Oscillatory Reversible Osmotic Growth of Sessile Saline Droplets on a Floating Polydimethylsiloxane Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
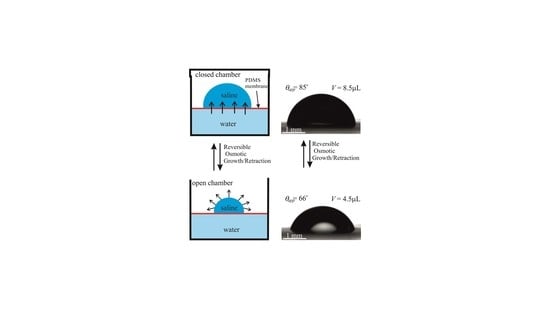
- In the long-time experiments, the stage of growth continued for . During this time, the droplet volume increased from 5 µL to 30 µL. Afterwards, the chamber was opened, as shown in Figure 2 and Video S1 and the droplet was evaporated during Increasing the evaporation time scale gave rise to the formation of the NaCl crystals in the vicinity of the triple line as shown in Figure 2. At this stage, the volume of the droplet was decreased, and the triple line retracted. We performed cycles of the long-time osmotic growth/evaporation of a droplet and observed that the process is reversible. Statistical scattering of the contact radius and volume of the droplet within growth/evaporation cycles were established as ±0.05 mm and ±0.3 μL, respectively.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitrovski, S.M.; Elliott, L.C.C.; Nuzzo, R.G. Microfluidic devices for energy conversion: Planar integration and performance of a passive, fully immersed H2−O2 fuel cell. Langmuir 2004, 20, 6974–6976. [Google Scholar] [CrossRef] [PubMed]
- Vankelecom, I.F.J.; de Kinderen, J.; Dewitte, B.M.; Uytterhoeven, J.B. Incorporation of hydrophobic porous fillers in PDMS membranes for use in pervaporation. J. Phys. Chem. 1997, 101, 5182–5185. [Google Scholar] [CrossRef]
- Li, L.; Xiao, Z.; Tan, S.; Pu, L.; Zhang, Z. Composite PDMS membrane with high flux for the separation of organics from water by pervaporation. J. Membr. Sci. 2004, 243, 177–187. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Qui, H.-Z.; Liao, K.-S.; Lue, S.J.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of UV-ozone treatment on poly(dimethylsiloxane) membranes: Surface characterization and gas separation performance. Langmuir 2010, 26, 4392–4399. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nour, M.; Berean, K.; Balendhran, S.; Ou, J.Z.; Du Plessis, J.; McSweeney, C.; Bhaskaran, M.; Sriram, S.; Kalantar-zadeh, K. CNT/PDMS composite membranes for H2 and CH4 gas separation. Int. J. Hydrog. Energy 2013, 38, 10494–10501. [Google Scholar] [CrossRef] [Green Version]
- Nour, M.; Berean, K.; Griffin, M.J.; Matthews, G.I.; Bhaskaran, M.; Sriram, S.; Kalantar-zadeh, K. Nanocomposite carbon-PDMS membranes for gas separation. Sens. Actuator B Chem. 2012, 161, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Perucho, P.; Robles, Á.; Durán, F.; Ferrer, J.; Seco, A. PDMS membranes for feasible recovery of dissolved methane from AnMBR effluents. J. Membr. Sci. 2020, 604, 118070. [Google Scholar] [CrossRef]
- Ataeivarjovi, E.; Tang, Z.; Chen, J. Study on CO2 Desorption Behavior of a PDMS–SiO2 Hybrid Membrane Applied in a Novel CO2 Capture Process. ACS Appl. Mater. Interfaces 2018, 10, 28992–29002. [Google Scholar] [CrossRef]
- Logemann, M.; Alders, M.; Wist, M.; Pyankova, V.; Krakau, D.; Gottschalk, D.; Wessling, M. Can PDMS membranes separate aldehydes and alkenes at high temperatures? J. Membr. Sci. 2020, 615, 118334. [Google Scholar] [CrossRef]
- Zheng, B.; Tice, J.D.; Roach, L.S.; Ismagilov, R.F. A droplet-based, composite PDMS/glass capillary microfluidic system for evaluating protein crystallization conditions by microbatch and vapor-diffusion methods with on-chip X-ray diffraction. Angew. Chem. Int. 2004, 43, 2508–2511. [Google Scholar] [CrossRef]
- Zheng, B.; Gerdts, C.J.; Ismagilov, R.F. Using nanoliter plugs in microfluidics to facilitate and understand protein crystallization. Curr. Opin. Struct. Biol. 2005, 15, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.K.; Legchenkova, I.; Shoval, S.; Dombrovsky, L.A.; Bormashenko, E. Osmotic evolution of composite liquid marbles. J. Colloid Interface Sci. 2021, 592, 167–173. [Google Scholar]
- Thangawng, A.L.; Ruoff, R.S.; Swartz, M.A.; Glucksberg, M.R. An ultra-thin PDMS membrane as a bio/micro–nano interface: Fabrication and characterization. Biomed. Microdevices 2007, 9, 587–595. [Google Scholar] [CrossRef]
- Koo, J.W.; Ho, J.S.; An, J.; Zhang, Y.; Chua, C.K.; Chong, T.H. A review on spacers and membranes: Conventional or hybrid additive manufacturing? Water Res. 2020, 188, 116497. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.-H.; Park, Y. Floating-on-water fabrication method for thin Polydimethylsiloxane membranes. Polymers 2019, 11, 1264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liao, W.; Wang, Y.; Wang, Y.; Wilson, D.I.; Clarke, S.M.; Yang, Z. The growth and shrinkage of water droplets at the oil-solid interface. J. Colloid Interface Sci. 2021, 584, 738–748. [Google Scholar] [CrossRef]
- Erbil, H.Y. Surface Chemistry of Solid and Liquid Interfaces; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Tadmor, R. Line energy and the relation between advancing, receding, and young contact angles. Langmuir 2004, 20, 7659–7664. [Google Scholar] [CrossRef]
- Tadmor, R. Approaches in wetting phenomena. Soft Matter 2011, 7, 1577–1580. [Google Scholar] [CrossRef]
- Tadmor, R.; Yadav, P.S. As-placed contact angles for sessile drops. J. Colloid Interface Sci. 2008, 317, 241–246. [Google Scholar] [CrossRef]
- Liu, J.; Xia, R.; Zhou, X. A new look on wetting models: Continuum analysis. Sci. China Phys. Mech. Astron. 2012, 55, 2158–2166. [Google Scholar] [CrossRef]
- Liu, J.; Mei, Y.; Xia, R. A new wetting mechanism based upon triple contact line pinning. Langmuir 2011, 27, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Physics of Wetting Phenomena and Applications of Fluids on Surfaces; Walter de Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Larson, R.G. Analysis of the effects of Marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef]
- Zhong, X.; Ren, J.; Duan, F.J. Wettability effect on evaporation dynamics and crystalline patterns of sessile saline droplets. J. Phys. Chem. B 2017, 121, 7924–7933. [Google Scholar] [CrossRef]
- Shao, X.; Duan, F.; Hou, Y.; Zhong, X. Role of surfactant in controlling the deposition pattern of a particle-laden droplet: Fundamentals and strategies. Adv. Colloid Interface Sci. 2020, 275, 102049. [Google Scholar] [CrossRef]
- Shao, Z.; Hou, Y.; Zhong, X. Modulation of evaporation-affected crystal motion in a drying droplet by saline and surfactant concentrations. Colloids Surfaces A 2021, 623, 126701. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, D.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, K. A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Efficient electrolytes for lithium–sulfur batteries. Front. Energy Res. 2015, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.K.; Shoval, S.; Dombrovsky, L.A.; Bormashenko, E. Oscillatory Reversible Osmotic Growth of Sessile Saline Droplets on a Floating Polydimethylsiloxane Membrane. Fluids 2021, 6, 232. https://doi.org/10.3390/fluids6070232
Roy PK, Shoval S, Dombrovsky LA, Bormashenko E. Oscillatory Reversible Osmotic Growth of Sessile Saline Droplets on a Floating Polydimethylsiloxane Membrane. Fluids. 2021; 6(7):232. https://doi.org/10.3390/fluids6070232
Chicago/Turabian StyleRoy, Pritam Kumar, Shraga Shoval, Leonid A. Dombrovsky, and Edward Bormashenko. 2021. "Oscillatory Reversible Osmotic Growth of Sessile Saline Droplets on a Floating Polydimethylsiloxane Membrane" Fluids 6, no. 7: 232. https://doi.org/10.3390/fluids6070232
APA StyleRoy, P. K., Shoval, S., Dombrovsky, L. A., & Bormashenko, E. (2021). Oscillatory Reversible Osmotic Growth of Sessile Saline Droplets on a Floating Polydimethylsiloxane Membrane. Fluids, 6(7), 232. https://doi.org/10.3390/fluids6070232