Rotational Maneuvers of Copepod Nauplii at Low Reynolds Number
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Speed Videography of Nauplii Swimming
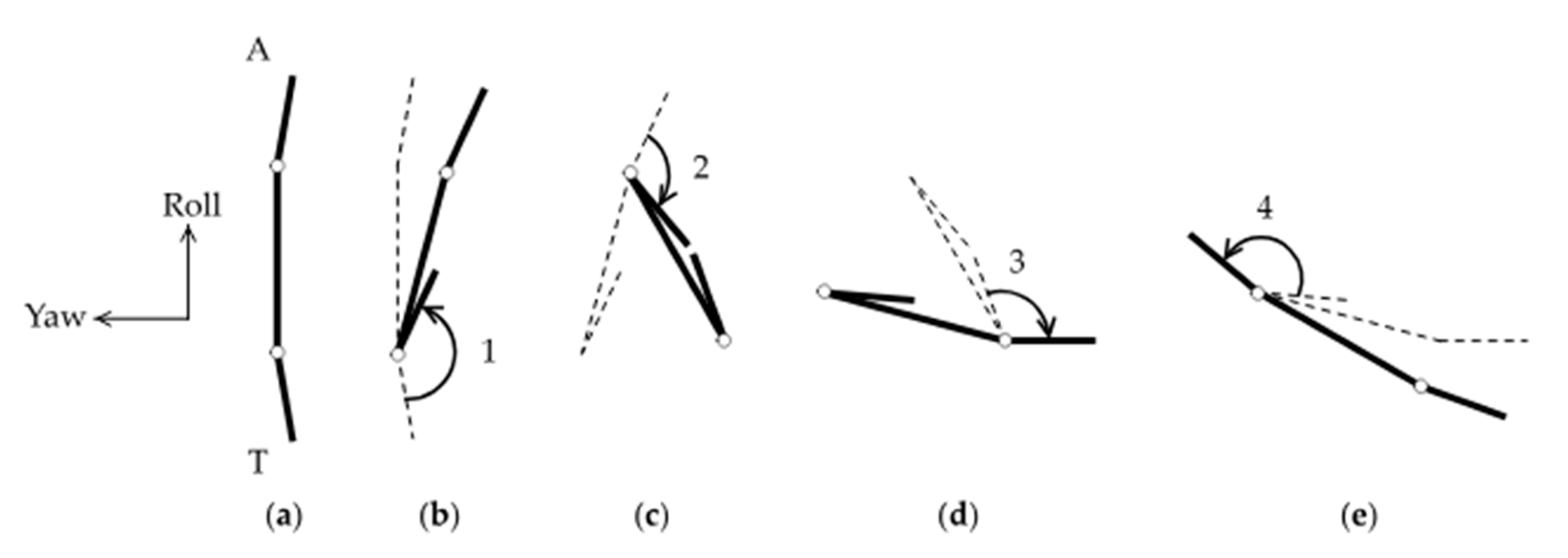
2.2. Video Analysis
3. Results
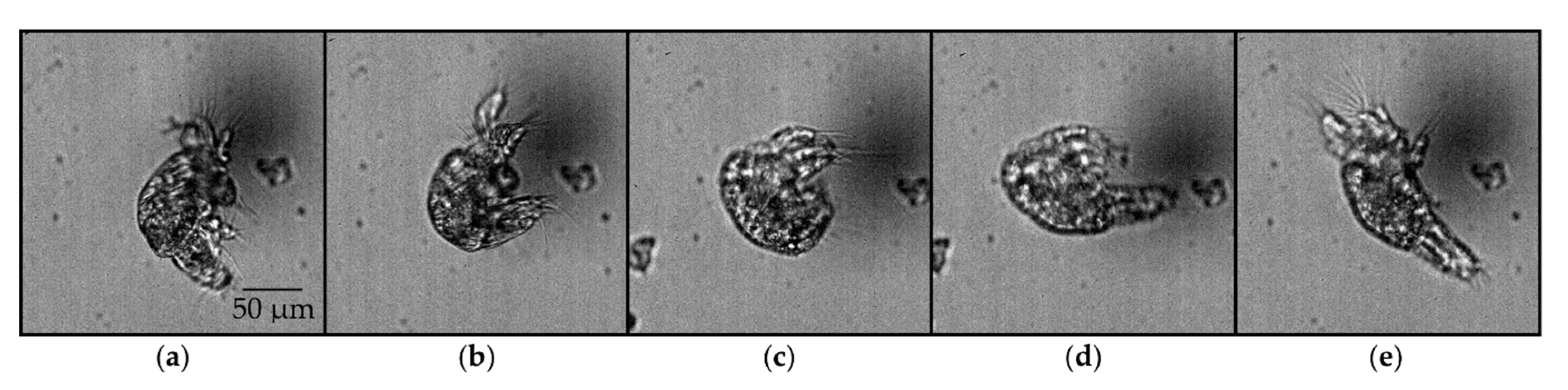
3.1. Overview
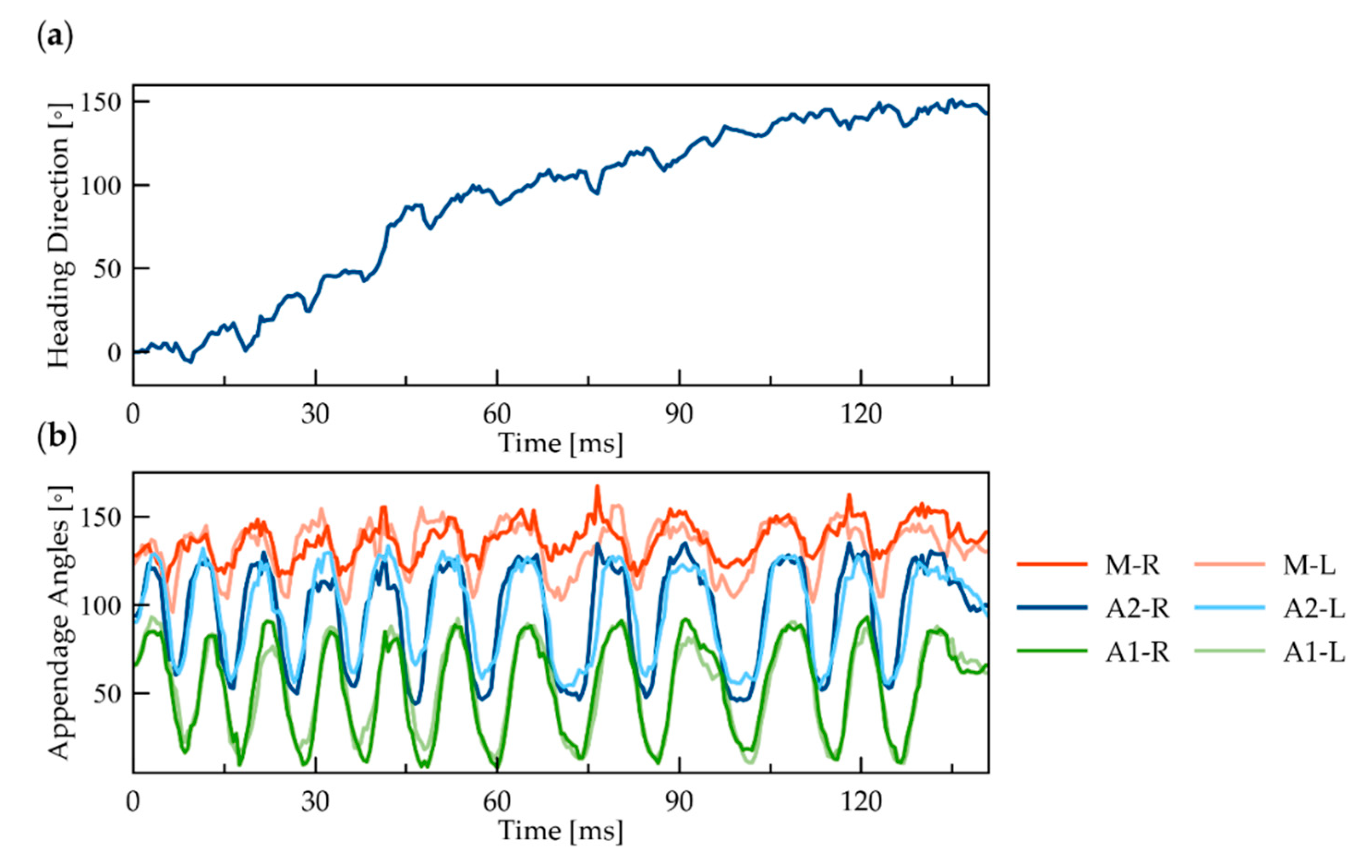
3.2. Yaw Rotation
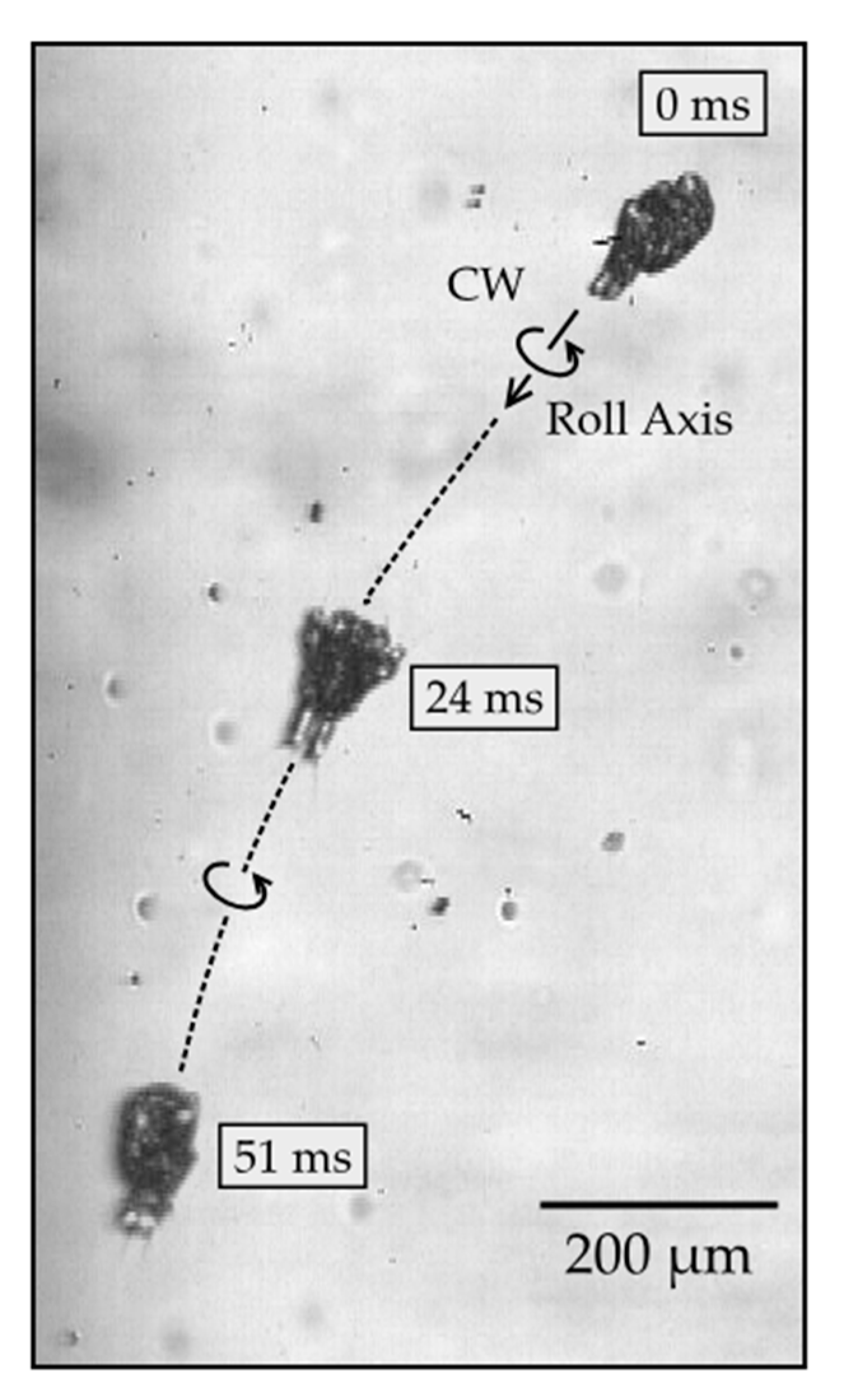
3.3. Roll Rotation
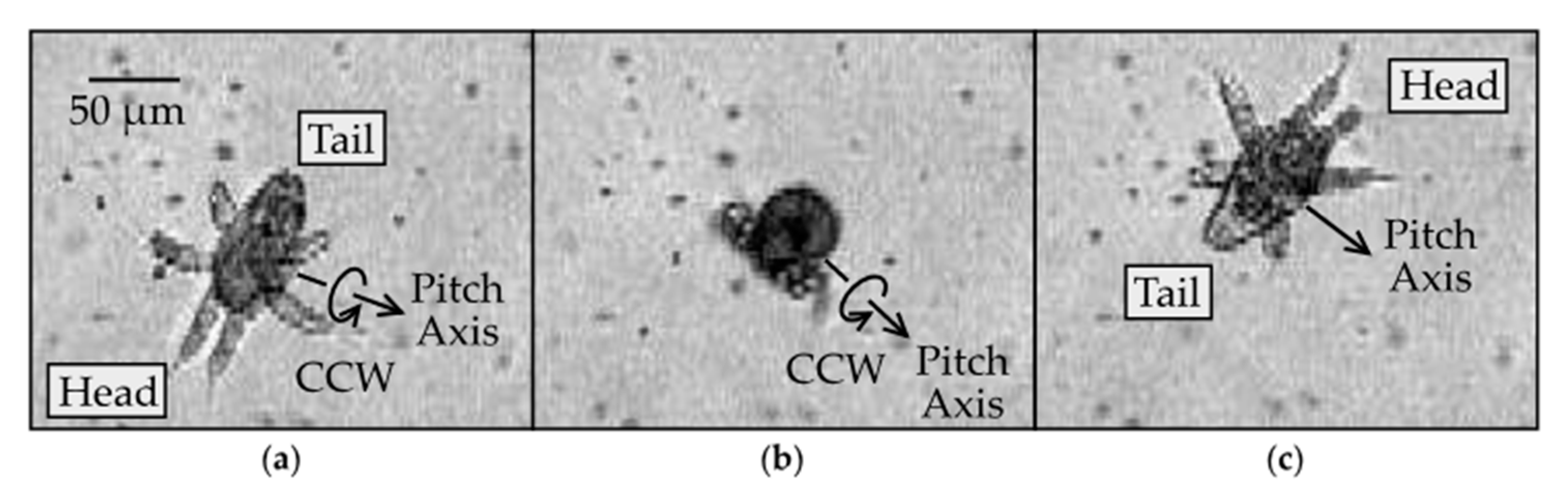
3.4. Pitch Rotation
3.5. Direction of Rotation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergou, A.J.; Ristroph, L.; Guckenheimer, J.; Cohen, I.; Wang, Z.J. Fruit flies modulate passive wing pitching to generate in-flight turns. Phys. Rev. Lett. 2010, 104, 148101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, F.E.; Nicastro, A.J.; Weihs, D. Dynamics of the aerial maneuvers of spinner dolphins. J. Exp. Biol. 2006, 209, 590–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauga, E. Bacterial hydrodynamics. Annu. Rev. Fluid Mech. 2016, 48, 105–130. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, R.E. Green algae as model organisms for biological fluid dynamics. Annu. Rev. Fluid Mech. 2015, 47, 343–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmell, B.J.; Sheng, J.; Buskey, E.J. Compensatory escape mechanism at low Reynolds number. Proc. Natl. Acad. Sci. USA 2013, 110, 4661–4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, P.H.; Takagi, D.; Hartline, D.K. Choreographed swimming of copepod nauplii. J. R. Soc. Interface 2015, 12, 20150776. [Google Scholar] [CrossRef] [Green Version]
- Purcell, E.M. Life at low Reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Dreyfus, R.; Baudry, J.; Stone, H.A. Purcell’s “rotator”: Mechanical rotation at low Reynolds number. Eur. Phys. J. B 2005, 47, 161–164. [Google Scholar] [CrossRef]
- Rizvi, M.S.; Farutin, A.; Misbah, C. Three-bead steering microswimmers. Phys. Rev. E 2018, 97, 023102. [Google Scholar] [CrossRef]
- Jalali, M.A.; Alam, M.R.; Mousavi, S. Versatile low-Reynolds-number swimmer with three-dimensional maneuverability. Phys. Rev. E 2014, 90, 053006. [Google Scholar] [CrossRef] [Green Version]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregón, A.; Nelson, B.J. Magnetic helical micromachines: Fabrication, controlled swimming, and cargo transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hosney, A.; Klingner, A.; Misra, S.; Khalil, I.S.M. Propulsion and steering of helical magnetic microrobots using two synchronized rotating dipole fields in three-dimensional space. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 1988–1993. [Google Scholar]
- Paffenhöfer, G.A.; Lewis, K.D. Feeding behavior of nauplii of the genus Eucalanus (Copepoda, Calanoida). Mar. Ecol. Prog. Ser. 1989, 57, 129–136. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A.; Strickler, J.R.; Lewis, K.D.; Richman, S. Motion behavior of nauplii and early copepodid stages of marine planktonic copepods. J. Plankton Res. 1996, 18, 1699–1715. [Google Scholar] [CrossRef] [Green Version]
- Titelman, J. Swimming and escape behavior of copepod nauplii: Implications for predator-prey interactions among copepods. Mar. Ecol. Prog. Ser. 2001, 213, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Bradley, C.J.; Strickler, J.R.; Buskey, E.J.; Lenz, P.H. Swimming and escape behavior in two species of calanoid copepods from nauplius to adult. J. Plankton Res. 2013, 35, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Eiane, K.; Aksnes, D.L.; Ohman, M.D.; Wood, S.; Martinussen, M.B. Stage-specific mortality of Calanus spp. under different predation regimes. Limnol. Oceanogr. 2002, 47, 636–645. [Google Scholar] [CrossRef]
- Turner, J.T. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Sampey, A.; McKinnon, A.D.; Meekan, M.G.; McCormick, M.I. Glimpse into guts: Overview of the feeding of larvae of tropical shorefishes. Mar. Ecol. Prog. Ser. 2007, 339, 243–257. [Google Scholar] [CrossRef]
- Svetlichnyy, L. Speed, force and energy expenditure in the movement of copepods. Oceanology 1987, 27, 497–502. [Google Scholar]
- Alcaraz, M.; Strickler, J.R. Locomotion in copepods: Pattern of movements and energetics of Cyclops. Hydrobiologia 1988, 167, 409–414. [Google Scholar] [CrossRef]
- Lenz, P.H.; Hartline, D.K. Reaction times and force production during escape behavior of a calanoid copepod, Undinula vulgaris. Mar. Biol. 1999, 133, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Buskey, E.J.; Lenz, P.H.; Hartline, D.K. Escape behavior of planktonic copepods in response to hydrodynamic disturbances: High speed video analysis. Mar. Ecol. Prog. Ser. 2002, 235, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Buskey, E.J.; Strickler, J.R.; Bradley, C.J.; Hartline, D.K.; Lenz, P.H. Escapes in copepods: Comparison between myelinate and amyelinate species. J. Exp. Biol. 2017, 220, 754–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, H.E.; Strickler, J.R.; Henderson, M.J.; Hartline, D.K.; Lenz, P.H. Predation strategies of larval clownfish capturing evasive copepod prey. Mar. Ecol. Prog. Ser. 2019, 614, 125–146. [Google Scholar] [CrossRef] [Green Version]
- Bruno, E.; Andersen Borg, C.M.; Kiørboe, T. Prey detection and prey capture in copepod nauplii. PLoS ONE 2012, 7, e47906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T.A. The nauplius larva of crustaceans: Functional diversity and the phylotypic stage. Integr. Comp. Biol. 1994, 34, 562–569. [Google Scholar] [CrossRef]
- Takagi, D. Swimming with stiff legs at low Reynolds number. Phys. Rev. E 2015, 92, 023020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, R.; Takagi, D. Metachronal swimming with rigid arms near boundaries. Fluids 2020, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- VanderLugt, K.; Lenz, P. Management of nauplius production in the paracalanid, Bestiolina similis (Crustacea: Copepoda): Effects of stocking densities and culture dilution. Aquaculture 2008, 276, 69–77. [Google Scholar] [CrossRef]
- Bathen, K.H. A Descriptive Study of the Physical Oceanography of Kaneohe Bay, Oahu, Hawaii; Hawai’i Institute of Marine Biology (Formerly Hawai’i Marine Laboratory): Kaneohe, HI, USA, 1968. [Google Scholar]
- Mauchline, J. The Biology of Calanoid Copepods; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Jennings, H.S. On the significance of the spiral swimming organisms. Am. Nat. 1901, 35, 369–378. [Google Scholar] [CrossRef]
- Crenshaw, H.C. A new look at locomotion in microorganisms: Rotating and translating. Integr. Comp. Biol. 1996, 36, 608–618. [Google Scholar] [CrossRef]
- Becker, L.E.; Koehler, S.A.; Stone, H.A. On self-propulsion of micro-machines at low Reynolds number: Purcell’s three-link swimmer. J. Fluid Mech. 2003, 490, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.A. A model of rowing propulsion and the ontogeny of locomotion in Artemia larvae. Biol. Bull. 1994, 187, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.U.; Fornshell, J.A.; Fornshell, B.J. Key for the identification of crustacean nauplii. Org. Divers. Evol. 2006, 6, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Titelman, J.; Kiørboe, T. Predator avoidance by nauplii. Mar. Ecol. Prog. Ser. 2003, 247, 134–149. [Google Scholar]
- Wadhwa, N.; Andersen, A.; Kiørboe, T. Hydrodynamics and energetics of jumping copepod nauplii and copepodids. J. Exp. Biol 2014, 217, 3085–3094. [Google Scholar]
- Mackas, D.L.; Tsuda, A. Mesozooplankton in the eastern and western subarctic Pacific: Community structure, seasonal life histories, and interannual variability. Prog. Oceangr. 1999, 43, 335–363. [Google Scholar] [CrossRef]
- Borg, M.A.; Bruno, E.; Kiørboe, T. The kinematics of swimming and relocation jumps in copepod nauplii. PLoS ONE 2012, 7, e47486. [Google Scholar] [CrossRef] [Green Version]



| Yaw | Roll | Pitch | |
|---|---|---|---|
| N1/N2 | 10 | 21 | 0 |
| ≥N3 | 15 | 19 | 30 |
| Yaw | Roll | Pitch | |
|---|---|---|---|
| CW | 10 | 12 | 0 |
| CCW | 8 | 14 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niimoto, K.T.M.; Kuball, K.J.; Block, L.N.; Lenz, P.H.; Takagi, D. Rotational Maneuvers of Copepod Nauplii at Low Reynolds Number. Fluids 2020, 5, 78. https://doi.org/10.3390/fluids5020078
Niimoto KTM, Kuball KJ, Block LN, Lenz PH, Takagi D. Rotational Maneuvers of Copepod Nauplii at Low Reynolds Number. Fluids. 2020; 5(2):78. https://doi.org/10.3390/fluids5020078
Chicago/Turabian StyleNiimoto, Kacie T. M., Kyleigh J. Kuball, Lauren N. Block, Petra H. Lenz, and Daisuke Takagi. 2020. "Rotational Maneuvers of Copepod Nauplii at Low Reynolds Number" Fluids 5, no. 2: 78. https://doi.org/10.3390/fluids5020078





