Agarose Cryogels: Production Process Modeling and Structural Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cryogel Formation
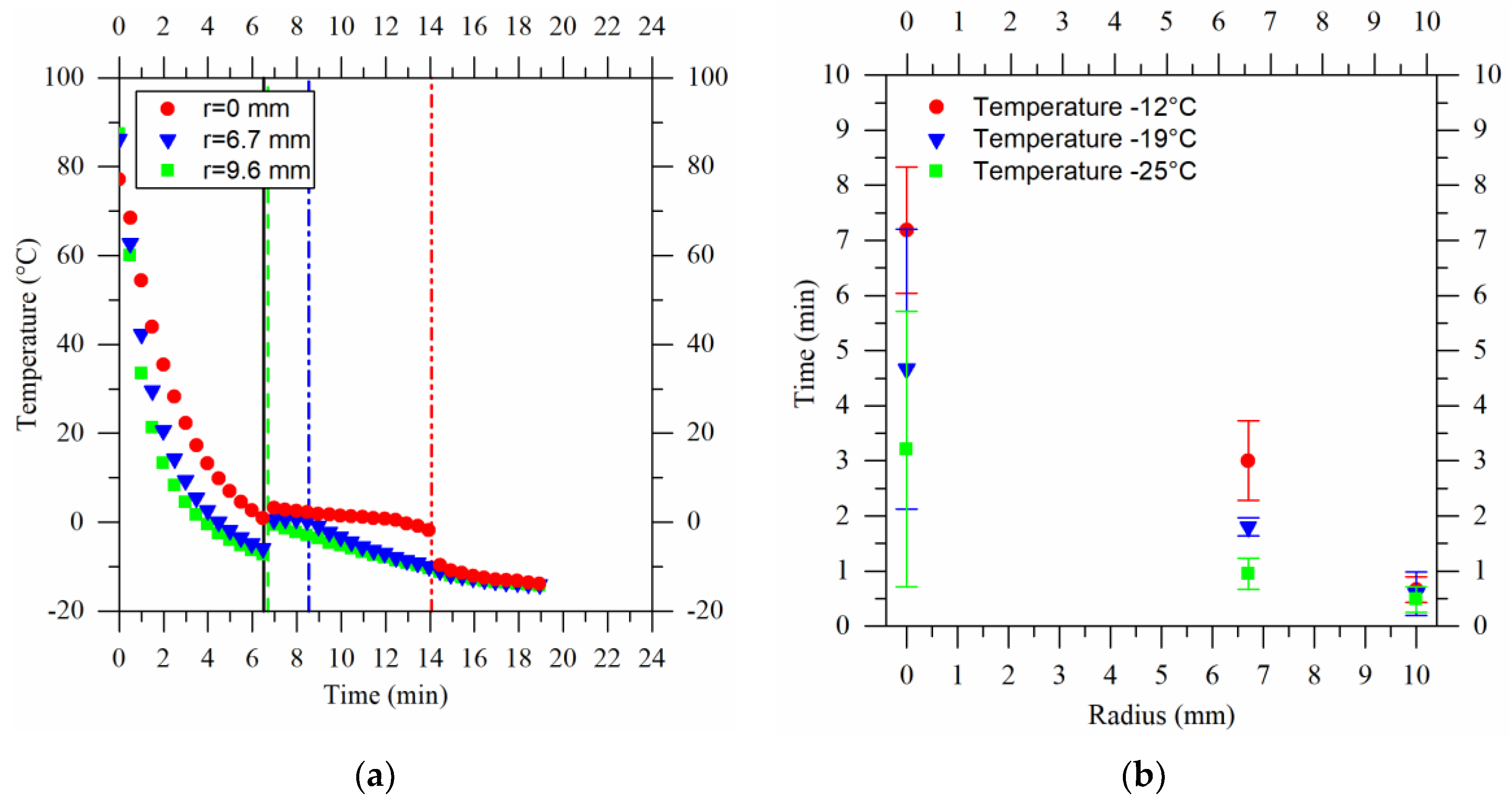
2.2. Process Temperature Modeling
2.3. Porosity Estimation of Cryogels
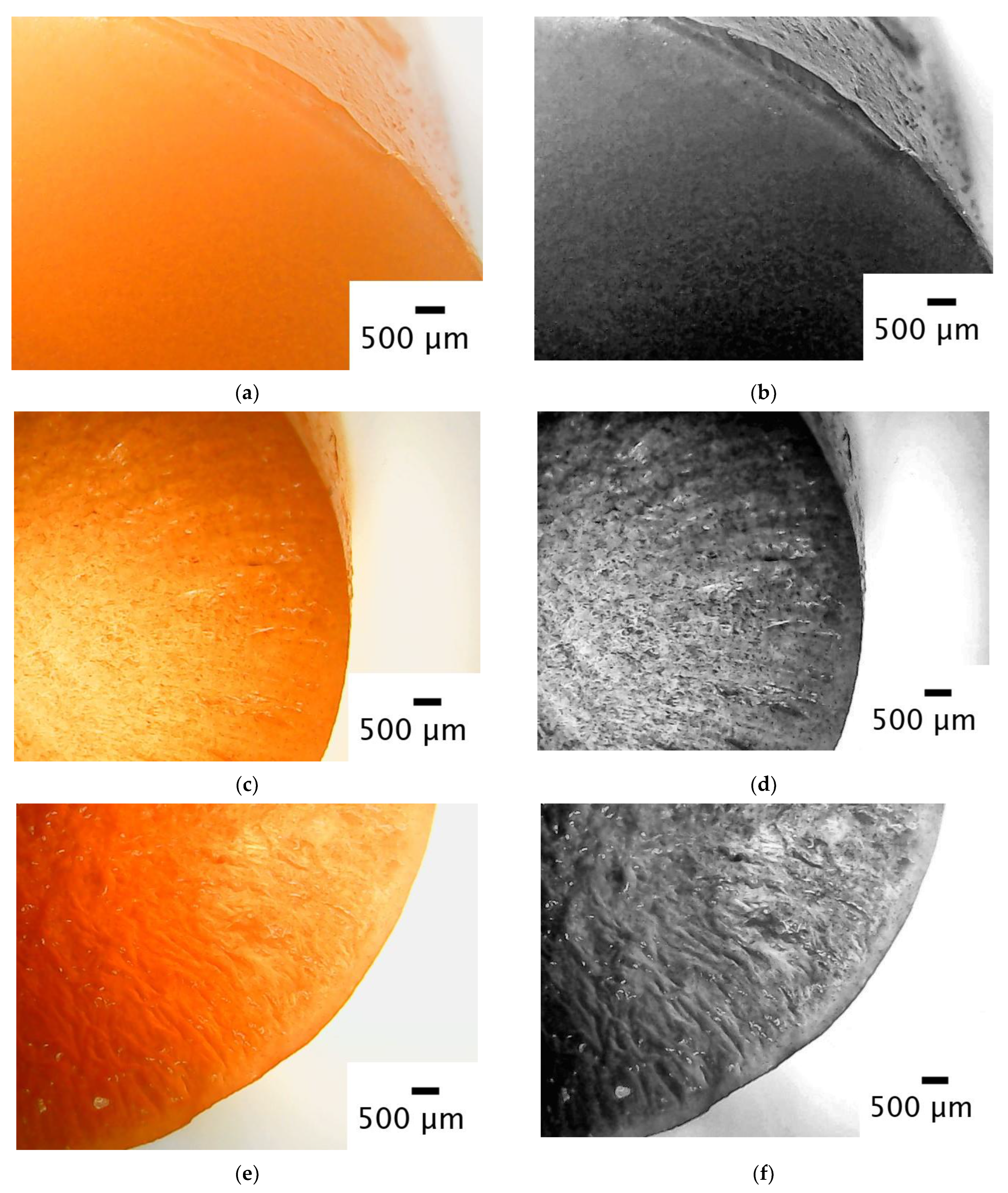
2.4. Mechanical Testing and Image Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Production Method of Agarose Gels
4.3. Thermal Profiles during the Formation of Cryogels
4.4. Mechanical Test and Image Analysis
5. Modeling
5.1. System Description
5.2. Properties of Materials
5.3. Model Equations
- looking at the boundary at r = 0, for each z, the axisymmetric condition was applied;
- a “Slip” condition was applied to the interface, so ; while on all other walls, u = 0;
- the initial velocity of the fluids was zero;
- the walls were adiabatic;
- a constant temperature of 15 °C was set on the wall and a constant temperature equal to the set point of the cooler, , was set on the wall;
- the known initial temperature, or the one measured at the start of the experiment, was set on the domains: = 10 °C, + 2 °C), = 25 °C, = 85 °C, = 25 °C, = 95 °C, and ;
- on the bottom of the chiller, a specific constraint was imposed on the pressure, specifying that it was constant and equal to the hydrostatic pressure , where is the gravitational acceleration and is the height;
- crystallization could not take place until the average crystallization start temperature was reached. This statement was appropriate because the temperature at which the crystallization began depended on many factors; it was not always the same and was difficult to predict. However, it is also true that, provided that only the operating conditions of the system were changed, it was possible to identify a temperature range in which crystallization occurred and hence the definition of ;
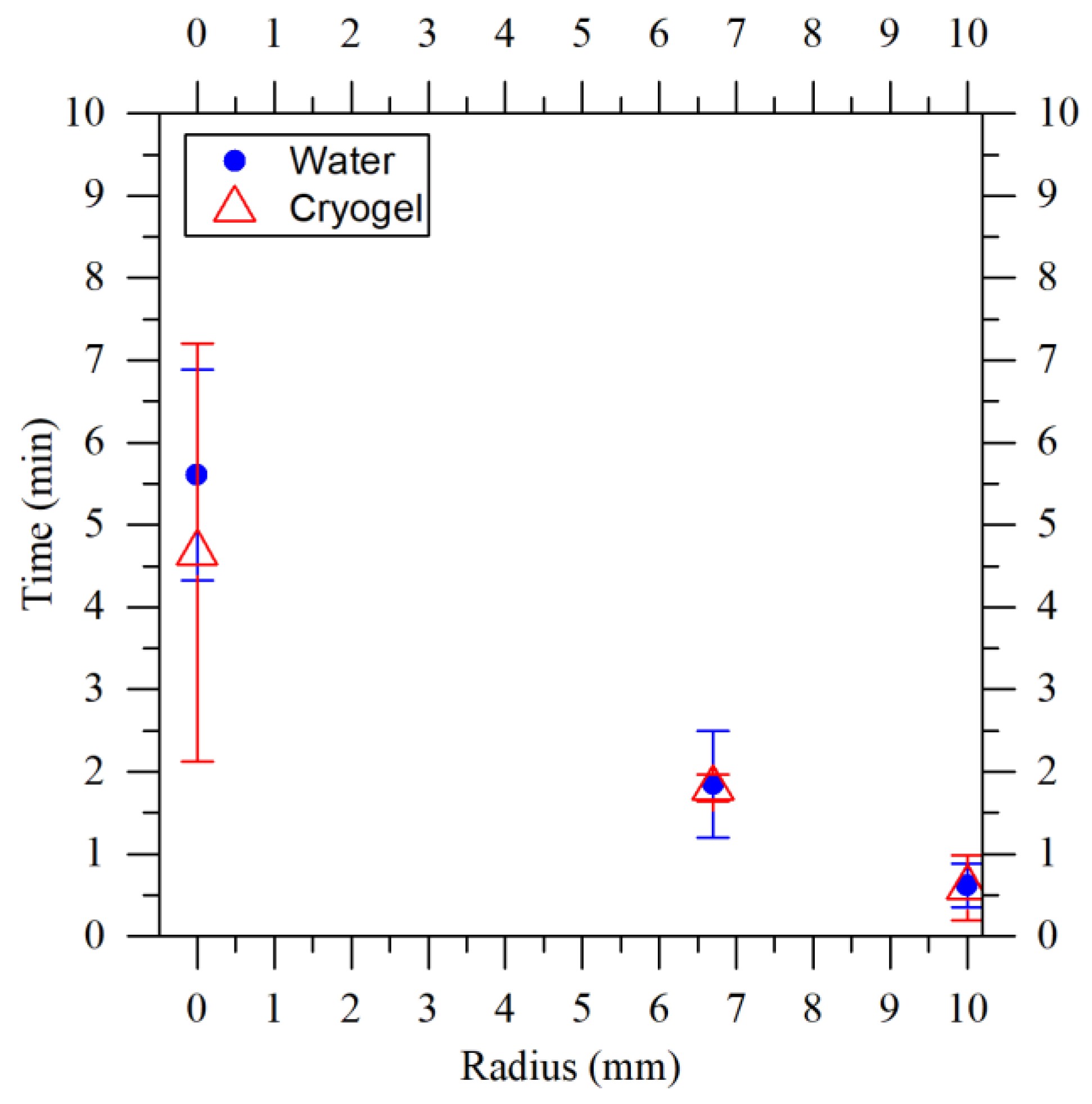
- the properties of the agarose cryogels were the same as water. Since the aim was to model the cryogel production process and the cryogels produced were 98% water, this assumption is reasonable. Moreover, the average freezing times of the water and agarose cryogels, obtained experimentally and shown in Figure 9, confirm this assumption. Here, the average freezing time was calculated as the difference between the time when the crystallization began and the time when the sample was completely frozen.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef]
- Caccavo, D.; Lamberti, G.; Barba, A.A. Mechanics and drug release from poroviscoelastic hydrogels: Experiments and modeling. Eur. J. Pharm. Biopharm. 2020, 152, 299–306. [Google Scholar] [CrossRef]
- Awadhiya, A.; Tyeb, S.; Rathore, K.; Verma, V. Agarose bioplastic-based drug delivery system for surgical and wound dressings. Eng. Life Sci. 2017, 17, 204–214. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 62, e3923. [Google Scholar]
- Guastaferro, M.; Baldino, L.; Reverchon, E.; Cardea, S. Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying. Gels 2021, 7, 198. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin–fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. Porous polymer monoliths: Amazingly wide variety of techniques enabling their preparation. J. Chromatogr. A 2010, 1217, 902–924. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Kirsebom, H.; Mattiasson, B. Preparation of macroporous cryostructurated gel monoliths, their characterization and main applications. J. Sep. Sci. 2011, 34, 2164–2172. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Ström, A.; Larsson, A.; Okay, O. Preparation and physical properties of hyaluronic acid-based cryogels. J. Appl. Polym. Sci. 2015, 132, 42194. [Google Scholar] [CrossRef]
- Caccavo, D.; Cavallo, R.; Abrami, M.; Grassi, M.; Barba, A.A.; Lamberti, G. Dynamometric measurements of hydrogels’ mechanical spectra. J. Appl. Polym. Sci. 2021, 138, 50702. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef]
- Halib, N.; Mohd Amin, M.C.I.; Ahmad, I.; Abrami, M.; Fiorentino, S.; Farra, R.; Grassi, G.; Musiani, F.; Lapasin, R.; Grassi, M. Topological characterization of a bacterial cellulose–acrylic acid polymeric matrix. Eur. J. Pharm. Sci. 2014, 62, 326–333. [Google Scholar] [CrossRef]
- Arsiccio, A.; Barresi, A.A.; Pisano, R. Prediction of ice crystal size distribution after freezing of pharmaceutical solutions. Cryst. Growth Des. 2017, 17, 4573–4581. [Google Scholar] [CrossRef]
- Yokoyama, F.; Achife, E.C.; Momoda, J.; Shimamura, K.; Monobe, K. Morphology of optically anisotropic agarose hydrogel prepared by directional freezing. Colloid Polym. Sci. 1990, 268, 552–558. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hottot, A.; Vessot, S.; Andrieu, J. Modeling of freezing step during freeze-drying of drugs in vials. AIChE J. 2007, 53, 1362–1372. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Voda, A.; Van Dalen, G.; Duijster, A. Ice crystal interspacing in frozen foods. J. Food Eng. 2013, 116, 622–626. [Google Scholar] [CrossRef]
- Janaky, N.; Jun-Ying, X.; Xiang-Yang, L. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006, 28, 83. [Google Scholar] [CrossRef]
- Jayawardena, I.; Turunen, P.; Garms, B.C.; Rowan, A.; Corrie, S.; Grøndahl, L. Evaluation of techniques used for visualisation of hydrogel morphology and determination of pore size distributions. Mater. Adv. 2023, 4, 669–682. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Bohne, D.; Fisher, S.; Obermeier, E. Thermal Conductivity, Density, Viscosity, and Prandtl-Numbers of Ethylene Glycol-Water Mixtures. Phys. Chem. 1984, 88, 739–742. [Google Scholar] [CrossRef]
- Toolbox, T.E. Engineering ToolBox-Resources, Tools and Basic Information for Engineering and Design of Technical Applications! Available online: https://www.engineeringtoolbox.com/ (accessed on 13 September 2023).
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill Professional Pub: New York, NY, USA, 2007. [Google Scholar]
- Nix, F.C.; MacNair, D. The thermal expansion of pure metals: Copper, gold, aluminum, nickel, and iron. Phys. Rev. 1941, 60, 597. [Google Scholar] [CrossRef]
- McBride, B.J. Coefficients for Calculating Thermodynamic and Transport Properties of Individual Species; National Aeronautics and Space Administration, Office of Management: Washington, DC, USA, 1993.
- Childs, G.E.; Ericks, L.J.; Powell, R.L. Thermal Conductivity of Solids at Room Temperature and Below: A Review and Compilation of the Literature; University of Michigan Library: Ann Arbor, MI, USA, 1973. [Google Scholar]
- Corruccini, R.J.; Gniewek, J.J. Thermal Expansion of Technical Solids at Low Temperatures: A Compilation from the Literature; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1961.
- Lucks, C.F.; Deem, H.W.; Wood, W.D. Thermal properties of six glasses and two graphites. Am. Ceram. Soc. Bull. 1960, 39. Available online: https://www.osti.gov/biblio/4152059 (accessed on 13 September 2023).
- Frei, W. Which Turbulence Model Should I Choose for My CFD Application? Available online: https://www.comsol.it/blogs/which-turbulence-model-should-choose-cfd-application/ (accessed on 13 September 2023).






| Variable | Unit of Measurement | Description |
|---|---|---|
| kg/m3 | Fluid density | |
| J/kg | Specific heat capacity of the solid phase | |
| J/kg | Specific heat capacity of the liquid phase | |
| J/kg | Specific heat capacity | |
| J/(m3·s) | Power per unit volume due to the latent heat of fusion | |
| J/(m3·s) | Power per unit volume due to nucleation | |
| K | Temperature of subcooled solution | |
| K | Temperature of fluid freezing front | |
| K | Interface temperature between the mold and the agarose solution | |
| kg/(m3·s) | Nucleation rate per unit volume | |
| - | Volume fraction of the solid phase | |
| - | Volume fraction of the liquid phase | |
| J/(m·K·s) | Thermal conductivity of the solid phase | |
| J/(m·K·s) | Thermal conductivity of the liquid phase | |
| kg/m3 | Density of the solid phase | |
| kg/m3 | Density of the liquid phase | |
| T | K | Temperature |
| Pa | Pressure | |
| s | Time | |
| m/s | Fluid velocity | |
| - | Identity matrix | |
| Pa | Stress tensor | |
| m2/s | Gravitational acceleration | |
| m | Height | |
| J/(m2·s) | Conductive heat flux | |
| J/(m·K·s) | Thermal conductivity | |
| J/kg | Latent heat due to phase change | |
| Pa·s | Fluid dynamic viscosity | |
| Pa·s | Turbulent fluid dynamic viscosity | |
| m2/s2 | Turbulent kinetic energy | |
| ε | m2/s3 | Turbulent kinetic dissipation rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancino, R.; Caccavo, D.; Barba, A.A.; Lamberti, G.; Biasin, A.; Cortesi, A.; Grassi, G.; Grassi, M.; Abrami, M. Agarose Cryogels: Production Process Modeling and Structural Characterization. Gels 2023, 9, 765. https://doi.org/10.3390/gels9090765
Mancino R, Caccavo D, Barba AA, Lamberti G, Biasin A, Cortesi A, Grassi G, Grassi M, Abrami M. Agarose Cryogels: Production Process Modeling and Structural Characterization. Gels. 2023; 9(9):765. https://doi.org/10.3390/gels9090765
Chicago/Turabian StyleMancino, Raffaele, Diego Caccavo, Anna Angela Barba, Gaetano Lamberti, Alice Biasin, Angelo Cortesi, Gabriele Grassi, Mario Grassi, and Michela Abrami. 2023. "Agarose Cryogels: Production Process Modeling and Structural Characterization" Gels 9, no. 9: 765. https://doi.org/10.3390/gels9090765





