Tissue Regeneration with Gelatine/Polysaccharide Derived Hydrogel Scaffolds: From Formulation to In Vivo Efficacy
Abstract
:1. Introduction
2. Results and Discussion
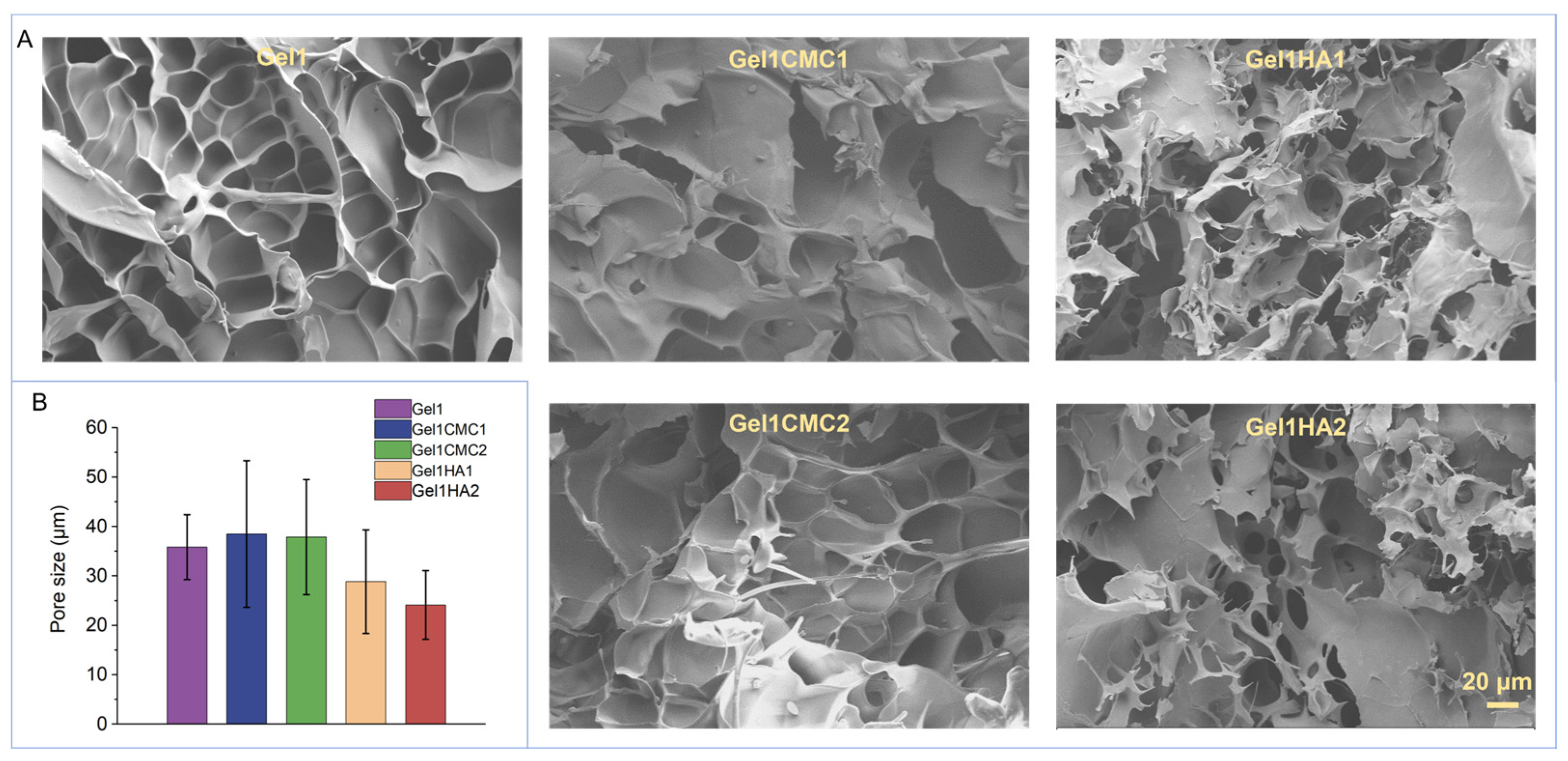
2.1. Fabrication of Gelatine-Based Hydrogel Scaffolds and Characterisation
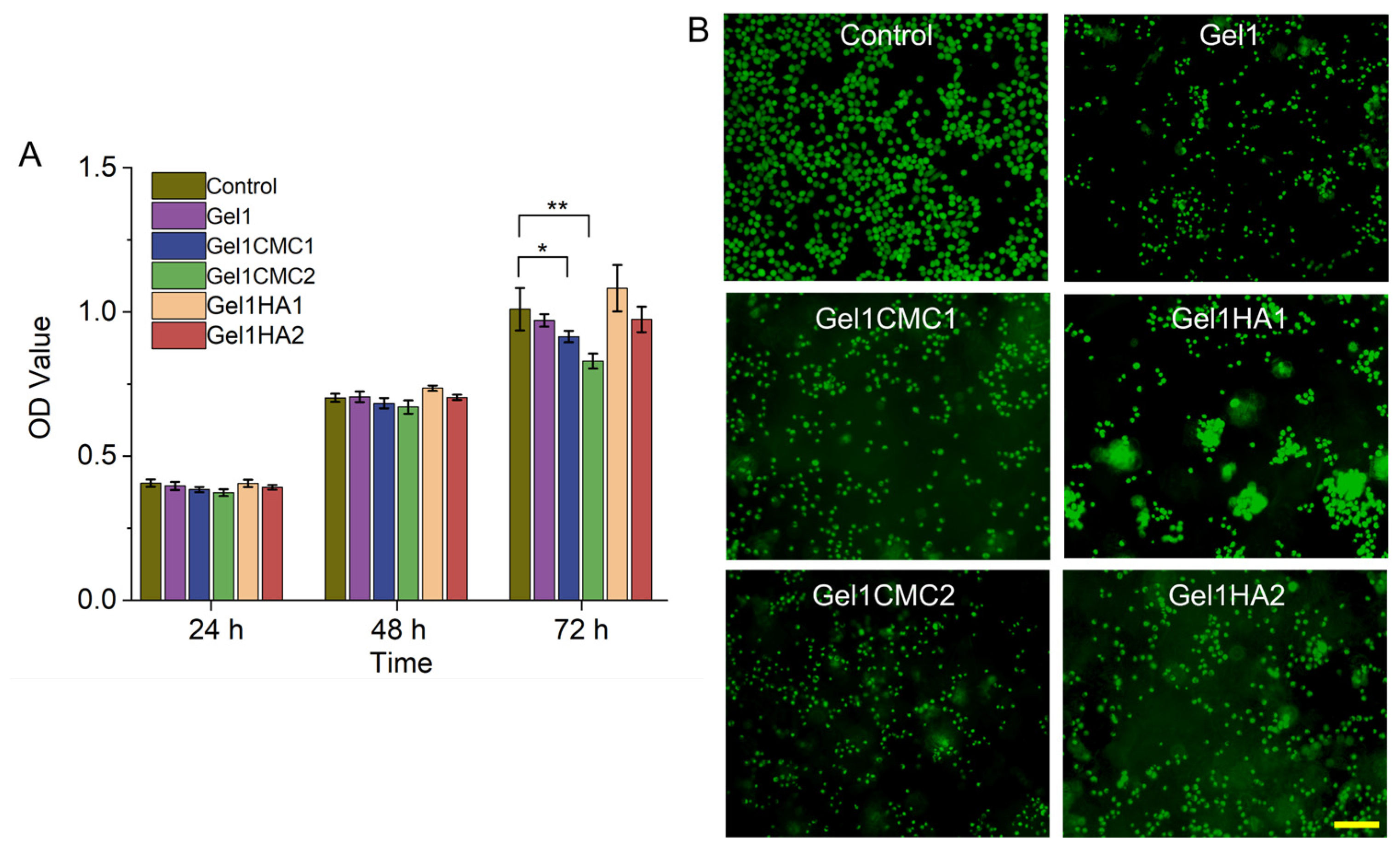
2.2. In Vitro Cytocompatibility Assessment
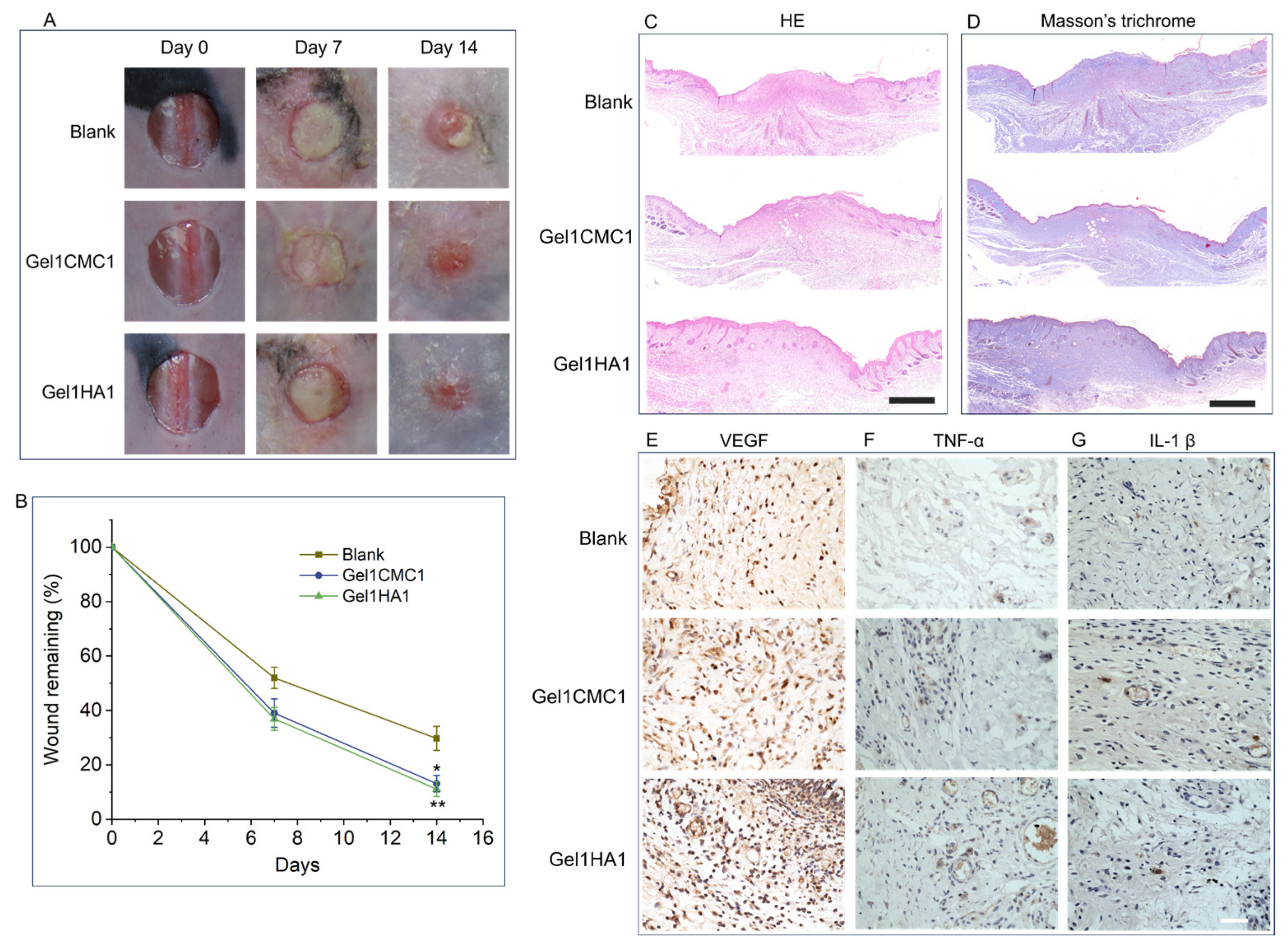
2.3. In Vivo Skin Wound Healing
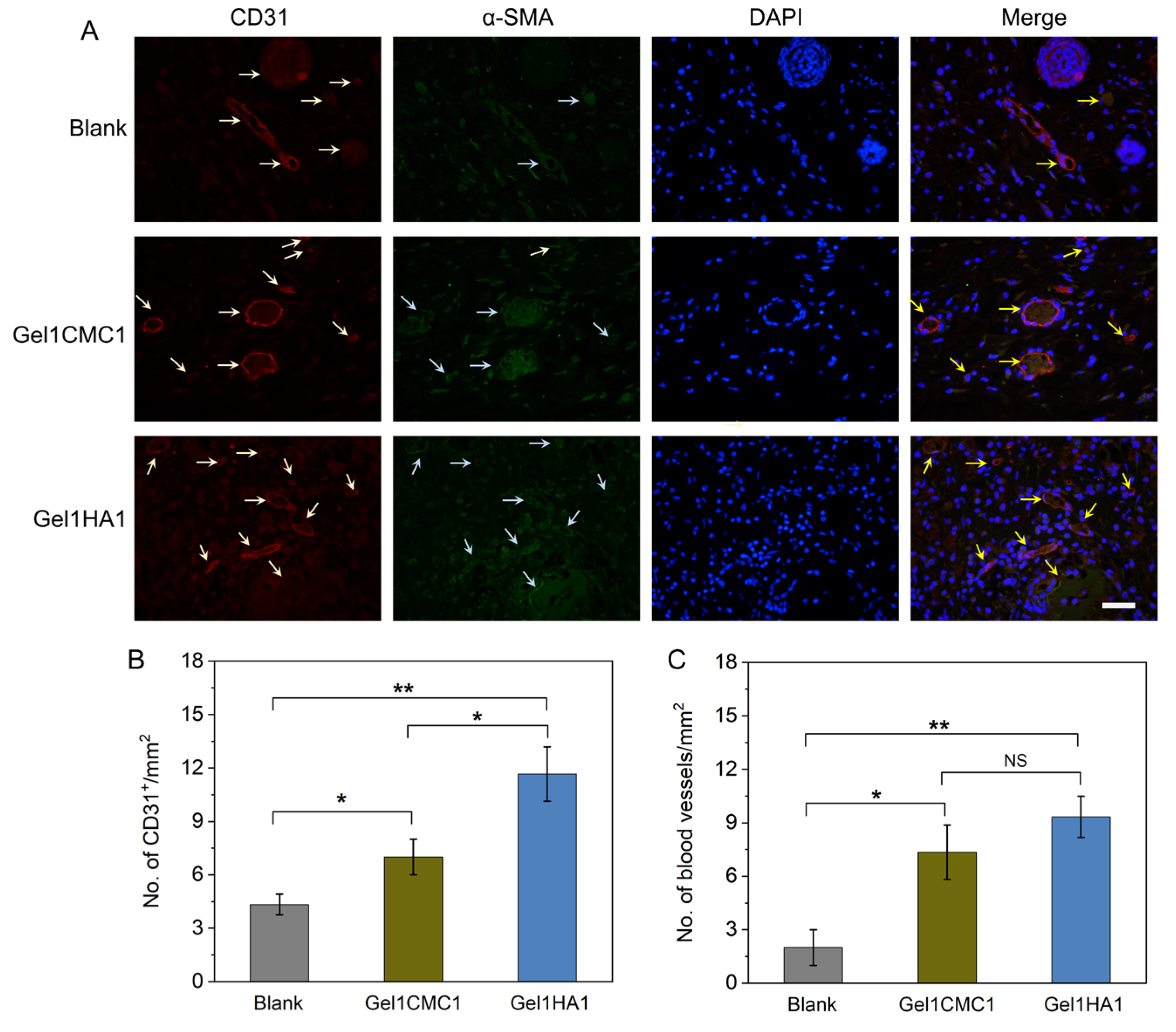
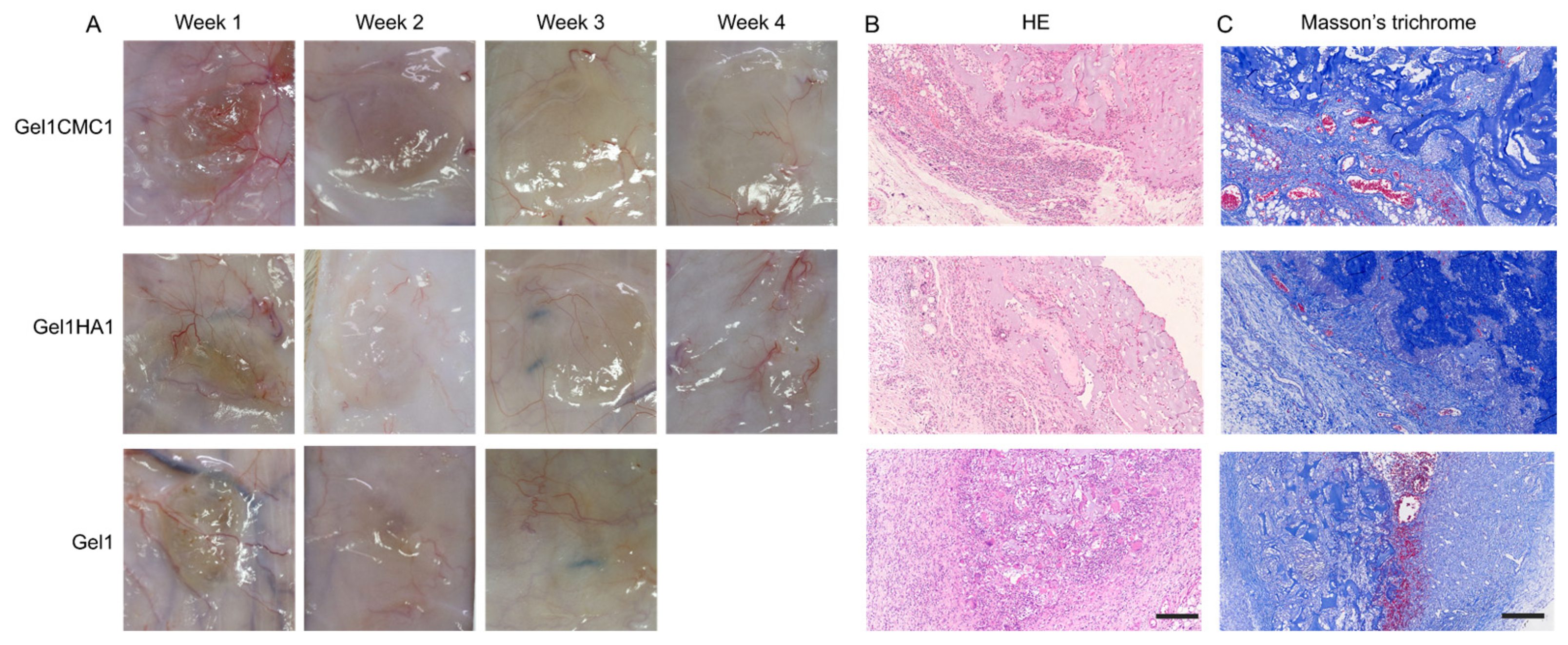
2.4. In Vivo Hydrogel Intergration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Different Scaffolds
4.3. Characterisation of the Hydrogel Scaffolds
4.4. Morphology Characterisation of the Hydrogel Scaffold
4.5. In Vitro Cell Proliferation
4.6. Animal Experiment
4.7. In Vivo Skin Wound Healing
4.8. In Vivo Tissue Integration and Vascularisation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, H.; Pan, S.; Yu, L.; Xu, T.; Chen, Y. Advanced Theragenerative Biomaterials with Therapeutic and Regeneration Multifunctionality. Adv. Funct. Mater. 2020, 30, 2002621. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for soft tissue repair and regeneration: A focus on italian research in the field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers 2021, 13, 2299. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, P.W. Europe PMC Funders Group Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066. [Google Scholar] [CrossRef]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Kim, G. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, N. Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation. Carbohydr. Polym. 2021, 257, 117617. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Sutjaritvorakul, T.; Wiwatwongwana, F.; Imsuwan, P.; Whalley, A.J.S.; Chutipaijit, S. Enzymatic degradation of modified gelatin and carboxymethyl cellulose scaffolds. Mater. Today Proc. 2018, 5, 14813–14817. [Google Scholar] [CrossRef]
- Kang, H.J.; Park, S.S.; Saleh, T.; Ahn, K.M.; Lee, B.T. In vitro and in vivo evaluation of Ca/P-hyaluronic acid/gelatin based novel dental plugs for one-step socket preservation. Mater. Des. 2020, 194, 108891. [Google Scholar] [CrossRef]
- Le Thi, P.; Son, J.Y.; Lee, Y.; Ryu, S.B.; Park, K.M.; Park, K.D. Enzymatically Crosslinkable Hyaluronic Acid-Gelatin Hybrid Hydrogels as Potential Bioinks for Tissue Regeneration. Macromol. Res. 2020, 28, 400–406. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- López-Gutierrez, J.; Ramos-Payán, R.; Ayala-Ham, A.; Romero-Quintana, J.G.; Castillo-Ureta, H.; Villegas-Mercado, C.; Bermúdez, M.; Sanchez-Schmitz, G.; Aguilar-Medina, M. Biofunctionalization of hydrogel-based scaffolds for vascular tissue regeneration. Front. Mater. 2023, 10, 1168616. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2015, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hauser, J.; Ring, A. Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research. Bioengineering 2022, 9, 298. [Google Scholar] [CrossRef]


| Hydrogel Name | Gel (% w/v) | CMC (% w/v) | HA (% w/v) |
|---|---|---|---|
| Gel1 | 1.58 | - | - |
| Gel1CMC1 | 1.58 | 0.1 | - |
| Gel1CMC2 | 1.58 | 0.2 | - |
| Gel1HA1 | 1.58 | - | 0.1 |
| Gel1HA2 | 1.58 | - | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; He, K.; Xu, Q. Tissue Regeneration with Gelatine/Polysaccharide Derived Hydrogel Scaffolds: From Formulation to In Vivo Efficacy. Gels 2023, 9, 744. https://doi.org/10.3390/gels9090744
Li J, He K, Xu Q. Tissue Regeneration with Gelatine/Polysaccharide Derived Hydrogel Scaffolds: From Formulation to In Vivo Efficacy. Gels. 2023; 9(9):744. https://doi.org/10.3390/gels9090744
Chicago/Turabian StyleLi, Jing, Keying He, and Qian Xu. 2023. "Tissue Regeneration with Gelatine/Polysaccharide Derived Hydrogel Scaffolds: From Formulation to In Vivo Efficacy" Gels 9, no. 9: 744. https://doi.org/10.3390/gels9090744





