Role of Chitosan Hydrogels in Clinical Dentistry
Abstract
:1. Introduction
2. Historical Perspective of Chitosan
3. Structure of Hydrogel Chitin
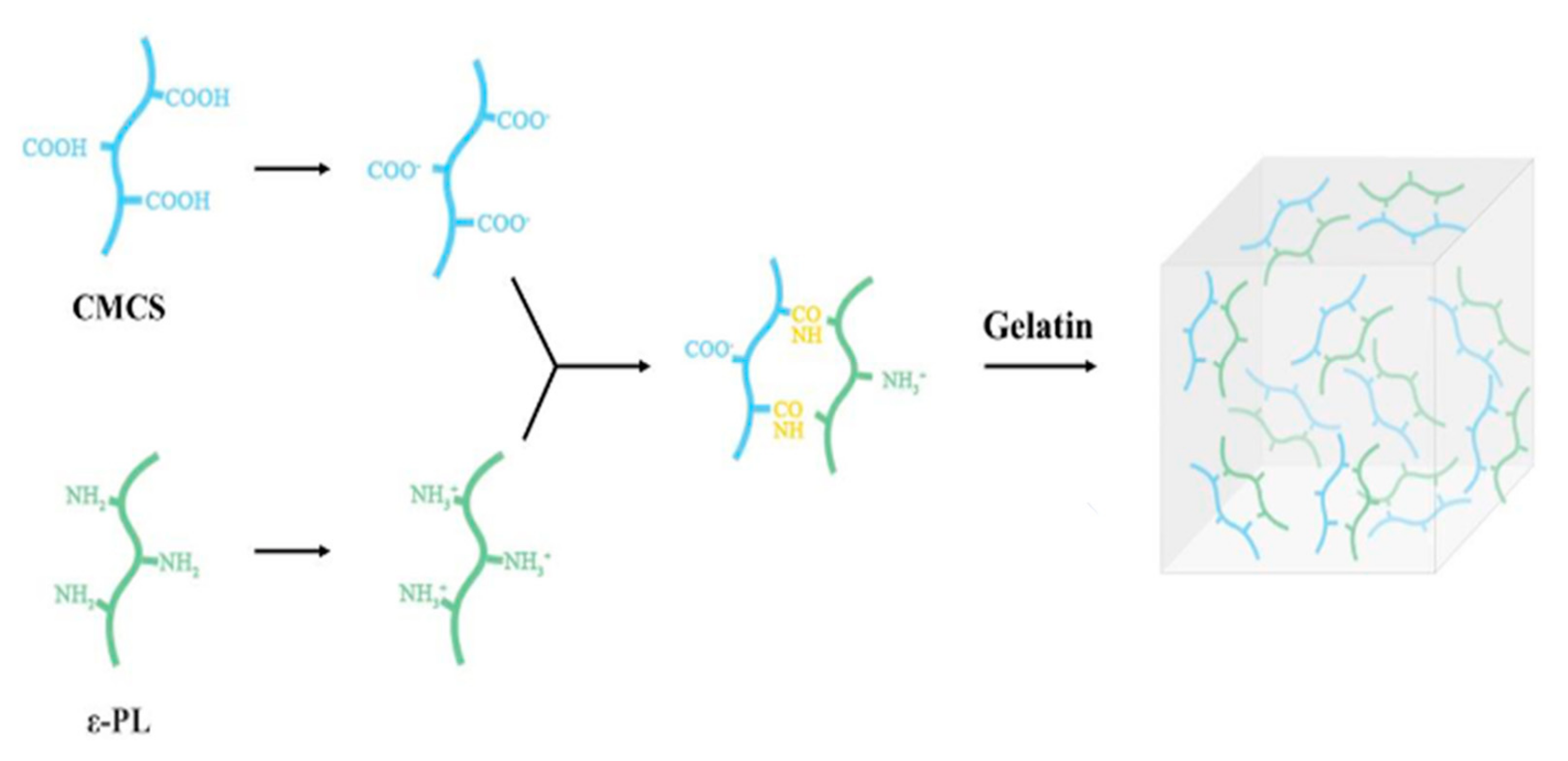
4. Production of Hydrogel Chitin
Properties of Chitosan
5. Applications in Dentistry
5.1. Preventive Dentistry
5.1.1. Dentifrices
5.1.2. Remineralization Potential
5.2. Restorative Dentistry
5.2.1. Hemostasis and Pulpotomy
5.2.2. Improvised GIC with Chitosan
5.2.3. Adhesion and Dentin Bonding
5.2.4. Regenerative Dentistry
6. Wound Healing
Implant Dentistry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Ma, L.; Mao, Z.; Gao, C. Chitosan-based biomaterials for tissue repair and regeneration. Chitosan for biomaterials II. In Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 81–127. [Google Scholar]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Blumenthal, H.J.; Roseman, S. Quantitative estimation of chitin in fungi. J. Bacteriol. 1957, 74, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, J.; Altenbach, H.; Malolepszy, T.; Mölleken, H. A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr. Res. 2011, 346, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of chitosan hydrogel for sustained delivery of VEGF for odontogenic differentiation of dental pulp stem cells. Stem Cells Int. 2019, 8, 2019. [Google Scholar] [CrossRef]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.C. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef]
- Singh, G.; Jamwal, U. Chitosan hydrogel: Its applications in medicine and dentistry. Int. J. Prev. Clin. Dent. Res. 2018, 5, 71. [Google Scholar]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and biological properties of a chitosan hydrogel scaffold associated to photobiomodulation therapy for dental pulp regeneration: An in vitro and in vivo study. BioMed Res. Int. 2021, 27, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Zhou, Y. Application progress of modified chitosan and its composite biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2022, 23, 6574. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, S.; Xie, C.; Wan, X.; Li, X.; Chen, K.; Zhao, G. Preparation of High Mechanical Strength Chitosan Nanofiber/NanoSiO2/PVA Composite Scaffolds for Bone Tissue Engineering Using Sol–Gel Method. Polymers 2022, 14, 2083. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Chitosan for biomaterials II. In Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–44. [Google Scholar]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. Part A 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Hoveizi, E.; Naddaf, H.; Ahmadianfar, S.; Gutmann, J.L. Encapsulation of human endometrial stem cells in chitosan hydrogel containing titanium oxide nanoparticles for dental pulp repair and tissue regeneration in male Wistar rats. J. Biosci. Bioeng. 2023, 135, 331–340. [Google Scholar] [CrossRef]
- Velasquez, C.L. Algunos usos del quitosano en sistemas acuosos. Rev. Iberoam. Polímeros 2003, 4, 91–109. [Google Scholar]
- Nadia, M.C.; Eric, L.; Giangiacomo, T.; Grégorio, C. Fundamentals and Applications of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123. [Google Scholar]
- Parhi, B.; Bharatiya, D.; Purohit, S.S.; Swain, S.K. Chitosan-Based Nano Biomaterials and Their Applications in Dentistry. In Chitosan Nanocomposites: Bionanomechanical Applications; Springer Nature: Singapore, 2023; pp. 325–348. [Google Scholar]
- Noohi, P.; Abdekhodaie, M.J.; Saadatmand, M.; Nekoofar, M.H.; Dummer, P.M. The development of a dental light curable PRFe-loaded hydrogel as a potential scaffold for pulp-dentine complex regeneration: An in vitro study. Int. Endod. J. 2023, 56, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Leite, Y.K.; Oliveira, A.C.; Quelemes, P.V.; Neto, N.M.; Carvalho, C.E.; Rodrigues, H.W.S.; Alves, M.M.; Carvalho, F.A.; Arcanjo, D.D.; Silva-Filho, E.C.; et al. Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells. Pharmaceuticals 2023, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Celesti, C.; Iannazzo, D.; Espro, C.; Visco, A.; Legnani, L.; Veltri, L.; Visalli, G.; Di Pietro, A.; Bottino, P.; Chiacchio, M.A. Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering. Materials 2022, 15, 8208. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Polysacch. I Struct. Charact. Use 2005, 186, 151–209. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chapter 31: Chitin and chitosan hydrogels. In Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 849–888. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Hafdani, F.N.; Sadeghinia, N. A Review on Application of Chitosan as a Natural Antimicrobial, World Academy of Science. Eng. Technol. 2011, 50, 252–256. [Google Scholar]
- Mohire, N.C.; Yadav, A.V. Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J. Dent. Res. 2010, 21, 380–384. [Google Scholar] [CrossRef]
- De Carvalho, M.; Stamford, T.; Pereira, E.; Dos Santos, P.; Sampaio, F. Chitosan as an oral antimicrobial agent. Formatex 2011, 2012, 13. [Google Scholar]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly(ethylene oxide) incorporating poly(hexamethylenebiguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bhise, S. Chitosan: A potential biomaterial effective against typhoid. Curr. Sci. 2004, 87, 1176–1178. [Google Scholar]
- Grobler, S.R.; Perchyonok, V.T.; Mulder, R.; Moodley, D. Towards Bioactive Dental Restorative Materials with Chitosan and Nanodiamonds: Evaluation and Application. Int. J. Dent. Oral Sci. 2015, 2, 147–154. [Google Scholar]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Liu, C.; Meng, X.; Lee, C.M.; Park, H. Preparation and biocompatibility of chitosan microcarriers as biomaterial. Biochem. Eng. J. 2006, 27, 269–274. [Google Scholar] [CrossRef]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.F. Chitosan-properties and applications in dentistry. Adv Tissue Eng. Regen. Med. Open Access 2017, 2, 205–211. [Google Scholar]
- Ganss, C.; Von Hinckeldey, J.; Tolle, A.; Schulze, K.; Klimek, J.; Schlueter, N. Efficacy of the stannous ion and a biopolymer in toothpastes on enamel erosion/abrasion. J. Dent. 2012, 40, 1036–1043. [Google Scholar] [CrossRef]
- Nimbeni, S.B.; Nimbeni, B.S.; Divakar, D.D. Role of Chitosan in Remineralization of Enamel and Dentin: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2021, 14, 562–568. [Google Scholar] [CrossRef]
- Schlueter, N.; Klimek, J.; Ganss, C. Randomised in situ study on the efficacy of a chitin/chitosan toothpaste on erosive-abrasive enamel loss. Caries Res. 2013, 47, 574–581. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Soleimani, A.A.; Rashidiani, J.; Malekafzali, B.; Abedini, F.; Hosseinkhani, H. Chitosan/fluoride nanoparticles for preventing dental caries. Curr. Dent. 2019, 1, 61–67. [Google Scholar] [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pavez, L.; Tobar, N.; Chacón, C.; Arancibia, R.; Martínez, C.; Tapia, C.; Pastor, A.; González, M.; Martínez, J.; Smith, P.C. Chitosan-triclosan particles modulate inflammatory signaling in gingival fibroblasts. J. Periodontal Res. 2018, 53, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Karaca, E.O.; Kuru, B.E.; Gursoy, H.; Haugen, H.J.; Wohlfahrt, J.C. Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone—A randomized, feasibility parallel-arm clinical trial. J. Periodontol. 2021, 93, 780–789. [Google Scholar] [CrossRef]
- Decker, E.M.; Weiger, R.; Weich, L.; Heide, P.E.; Brecx, M. Comparision of antiadhesive and antibacterial effects of antiseptics on Streptococcus sanguinis. Eur. J. Oral Sci. 2003, 111, 144–148. [Google Scholar] [CrossRef]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofiber mats for dental caries prevention. Carbohydr. Polym. 2015, 6, 933–940. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yanagiguchi, K.; Yamada, S.; Ohara, N.; Ikeda, T.; Hayashi, Y. Chitosan monomer promotes tissue regeneration on dental pulp wounds. J. Biomed. Mater. Res. Part A 2006, 76, 711–720. [Google Scholar] [CrossRef]
- Hamilton, M.F.; Otte, A.D.; Gregory, R.L.; Pinal, R.; Ferreira-Zandona, A.; Bottino, M.C. Physicomechanical and antibacterial properties of experimental resin-based dental sealants modified with nylon-6 and chitosan nanofibers. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1560–1568. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, Y.M.; Park, S.N.; Sheen, S.Y.; Chung, C.P.; Lee, S.J. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials 2000, 21, 153–159. [Google Scholar] [CrossRef]
- ElShiha, H.Y.; Tawfik, H.A.M.; AbouSamrah, N.K.; Marzouk, H.A.E.M. Efficacy of chitosan and absorbable gelatin sponge on hemostasis and wound healing following tooth extraction “A Comparative Study”. Egypt. Dent. J. 2012, 58, 1–5. [Google Scholar]
- Malmquist, J.P.; Clemens, S.C.; Oien, H.J.; Wilson, S.L. Hemostatic of oral surgery wounds with the HemCon dental dressing. J. Oral Maxillofac. Surg. 2008, 66, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Tavaria, F.K.; Costa, E.M.; Pina-Vaz, I.; Carvalho, M.F.; Pintado, M.M. A quitosanacomo biomaterial odontológico: Estado da arte. Rev. Bras. Eng. Biomédica 2013, 29, 110–120. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K.; Sato, M.; Nakanishi, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Fujita, M.; Kikuchi, M.; et al. Acceleration of wound contraction and healing with a photocrosslinkable chitosan hydrogel. Wound Repair Regen. 2001, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Yazdanpanah, Z.; Papagerakis, S.; Chen, X.; Papagerakis, P. Self-crosslinkable oxidized alginate-carboxymethyl chitosan hydrogels as an injectable cell carrier for in vitro dental enamel regeneration. J. Funct. Biomater. 2022, 13, 71. [Google Scholar] [CrossRef]
- Sun, Y.; Miao, T.; Wang, Y.; Wang, X.; Lin, J.; Zhao, N.; Hu, Y.; Xu, F.J. A natural polyphenol-functionalized chitosan/gelatin sponge for accelerating hemostasis and infected wound healing. Biomater. Sci. 2023, 11, 2405–2418. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Micro. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Choi, K.S.; Ji, M.-K.; Kim, S.; Gwon, Y.; Park, C.; Kim, J.; Lim, H.-P. Graphene–Chitosan Hybrid Dental Implants with Enhanced Antibacterial and Cell-Proliferation Properties. Appl. Sci. 2020, 10, 4888. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in fabrication and application of chitosan composites in implants and dentistry: A review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Hallmann, L.; Gerngroß, M.D. Chitosan and its application in dental implantology. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e701–e707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Li, P.; Ning, N. The Effect of Chitosan in Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2021, 34, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cao, C.; Wang, A.; Zhao, Y.; Jin, M.; Wang, Y.; Chen, S.; Yu, M.; Yang, Z.; Qu, X.; et al. Injectable Double-Network Hydrogel-Based Three-Dimensional Cell Culture Systems for Regenerating Dental Pulp. ACS Appl. Mater. Interfaces 2023, 15, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, I.; Rathinavelu, P.K.; Arumugham, M.I.; Srisakthi, D.; Balasubramaniam, A. Efficacy of Chitosan and Chlorhexidine Mouthwash on Dental Plaque and Gingival Inflammation: A Systematic Review. Cureus 2022, 14, e23318. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Ramírez, J.M. Systematic review of effectiveness of chitosan as a biofunctionalizer of titanium implants. Biology 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.V.; Oliveira, M.J.; Barbosa, M.A.; Goncalves, R.M.; Castro, F. Harnessing chitosan and poly-(γ-glutamic acid)-based biomaterials towards cancer immunotherapy. Mat. Today Adv. 2022, 15, 100252. [Google Scholar] [CrossRef]
| Serial No. | Dental Arena | Chitosan Application | References |
|---|---|---|---|
| 1 | Preventive dentistry | Dentifrices, mucoadhesives, mouthwashes, antimicrobial agents, oral drug delivery | [26,27,28,36] |
| 2 | Conservative dentistry | Indirect pulp capping, direct pulp capping, pulpotomy, component of canal medicaments, sealants, enamel repair and remineralization, bonding agents | [5,7,29,30,41] |
| 3 | Surgery | Guided bone regeneration, hemostasis, bone tissue engineering, scaffolds | [35,36,42,43,44,45] |
| 4 | Implants | Titanium coatings along with chitosan, GC-based implants, bone regeneration around implants, peri-implantitis | [7,46,47,48,49,50,51] |
| 5 | Wound healing | Immune-modulators, gauze dressings | [2,4,36,43,44,45] |
| S. No | Authors Name | Type of Studies Included | No. of Studies Included | Important Findings |
|---|---|---|---|---|
| 1 | Cicciù, M., Fiorillo, L., & Cervino, G. 2019 [65] | Randomized controlled trials | 12 | Chitosan serves a variety of purposes, and it is employed in several dental specialties in a secure and efficient manner. Chitosan has a number of functions, including its ability to remineralize tooth tissue and, as a result, play the role of a desensitizer in toothpaste. Our comprehensive review found that using chitosan improved the surgical healing of oral ulcers sustained during tooth extraction. Additionally, when utilized in dental cement, some studies indicate a decrease in bacterial biofilm. Additionally, it has systemic qualities that make it useful for medication delivery, including antibacterial, antifungal, hemostatic, and other properties. |
| 2 | Liu, Ying M.S.; Chen, JiaLi M.S., R.N.; Li, PeiFang M.S.N., R.N.; Ning, Ning M.S., R.N. 2021 [1] | Randomized controlled trials | 5 | There have been more tests of novel chitosan dressings. However, there haven’t been many studies on how chitosan affects wound healing. According to recent research, chitosan does not impede the healing of wounds. The limited number of trials, however, made it difficult to interpret the data properly. To validate any clinically significant effect of chitosan on wound healing, further study must be carefully planned. |
| 3 | Pandiyan, I., Rathinavelu, P., Arumugham, M.I., et al. 2021 [68] | Randomized controlled trials | 3 | The most efficient chemical method of preventing plaque is mouthwash, which is used everywhere. Possible adverse effects include darkening of the teeth and tongue, a brief alteration in taste perception, a rise in calculus deposits, a burning sensation, and genotoxicity of buccal epithelial cells. In this review, the effectiveness of chitosan mouthwash in preventing plaque buildup and gingival irritation. |
| 4 | López-Valverde, N., López-Valverde, A., Ramírez, J.M. 2021 [69] | In vivo studies;Studies where at least one layer of CS was used to coat the Ti;Studies where bone growth or the formation of a biological seal around the Ti implant surface coated with CS alone or in combination with other products or molecules was assessed; Studies on endosseous implants; Studies that included non-modified animals (osteoporotics, diabetics…) | 7 | Ti dental implants with CS coating may be more capable of osseointegrating. The biofunctionalization of dental implants is probably going to become a commercial option in the future. However, to support the use of CS as a coating for Ti implants for osteoinduction purposes and subsequently to provide surfaces that ensure rapid osseointegration, confirmation of this possibility would require well-designed clinical research using broad samples, standardised protocols, and long-term monitoring.Ti dental implants with CS coating may be more capable of osseointegrating. The biofunctionalization of dental implants is probably going to become a commercial option in the future. To justify the use of CS as a coating, however, proof of this possibility would require well-designed clinical study involving large samples, standardised techniques, and long-term monitoring. |
| 5 | Lima, B.V., Oliveira, M.J., Barbosa, M.A., Gonçalves, R.M., & Castro, F. 2021 [70] | in vitro, in vivo and clinical studies which used Ch-based formulations and evaluated their ability to induce immune cell stimulation in the cancer context. | 57 | In general, Ch-based formulations reduce the number of cells that have anti-inflammatory effects while increasing the recruitment and proliferation of cells linked to pro-inflammatory properties. These outcomes were associated with a smaller tumour, fewer metastases, reversal of the immunosuppressive TME, and improved in vivo survival. Ch-based formulations, in general, present the possibilities for cancer immunotherapy. Clinical translation is still difficult, though, as most studies combine Ch with other ingredients, suggesting that part of the observed effects may be the consequence of the interaction of the separate effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, S.; Das, G.; Alqarni, M.; Grover, V.; Manzoor Baba, S.; Saluja, P.; Hassan, S.A.B.; Abdulla, A.M.; Bavabeedu, S.S.; Abullais, S.S.; et al. Role of Chitosan Hydrogels in Clinical Dentistry. Gels 2023, 9, 698. https://doi.org/10.3390/gels9090698
Arora S, Das G, Alqarni M, Grover V, Manzoor Baba S, Saluja P, Hassan SAB, Abdulla AM, Bavabeedu SS, Abullais SS, et al. Role of Chitosan Hydrogels in Clinical Dentistry. Gels. 2023; 9(9):698. https://doi.org/10.3390/gels9090698
Chicago/Turabian StyleArora, Suraj, Gotam Das, Mohammed Alqarni, Vishakha Grover, Suheel Manzoor Baba, Priyanka Saluja, Saeed Awod Bin Hassan, Anshad M. Abdulla, Shashit Shetty Bavabeedu, Shahabe Saquib Abullais, and et al. 2023. "Role of Chitosan Hydrogels in Clinical Dentistry" Gels 9, no. 9: 698. https://doi.org/10.3390/gels9090698







