Xanthan-Gum/Pluronic-F-127-Based-Drug-Loaded Polymeric Hydrogels Synthesized by Free Radical Polymerization Technique for Management of Attention-Deficit/Hyperactivity Disorder
Abstract
:1. Introduction
2. Results and Discussion
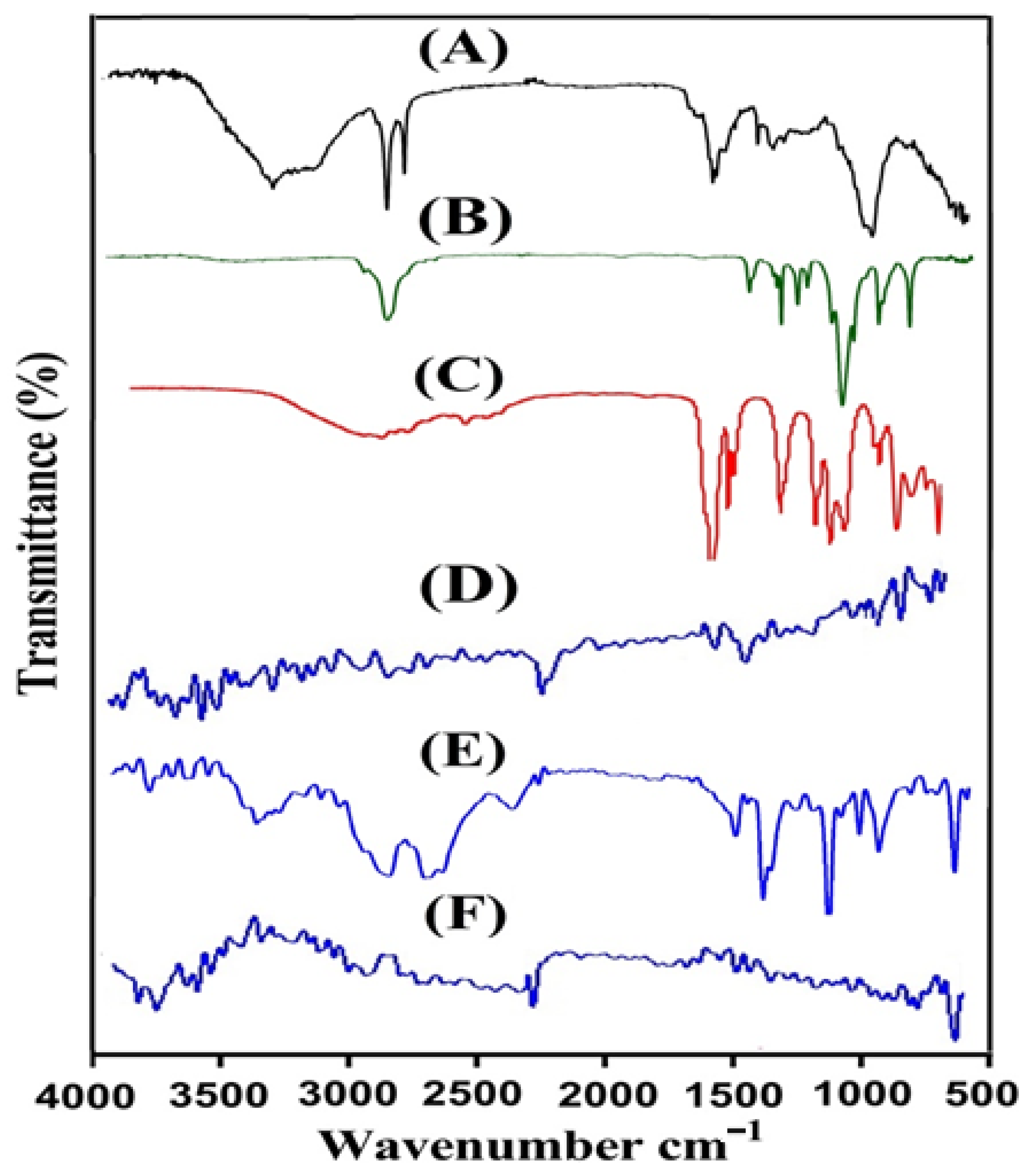
2.1. FTIR Analysis
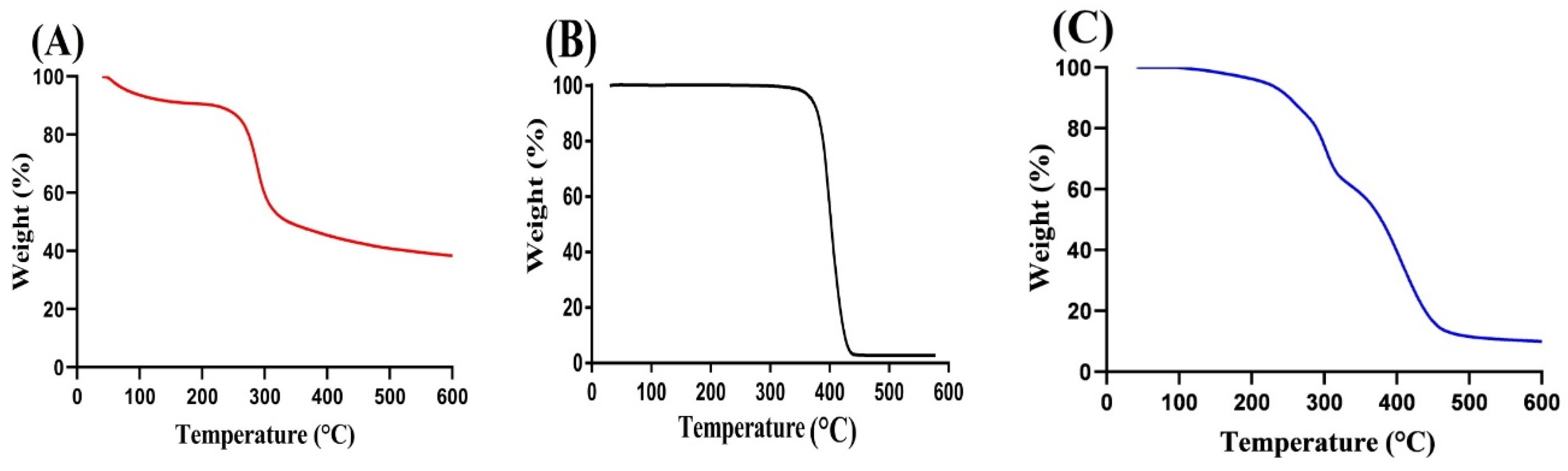
2.2. TGA
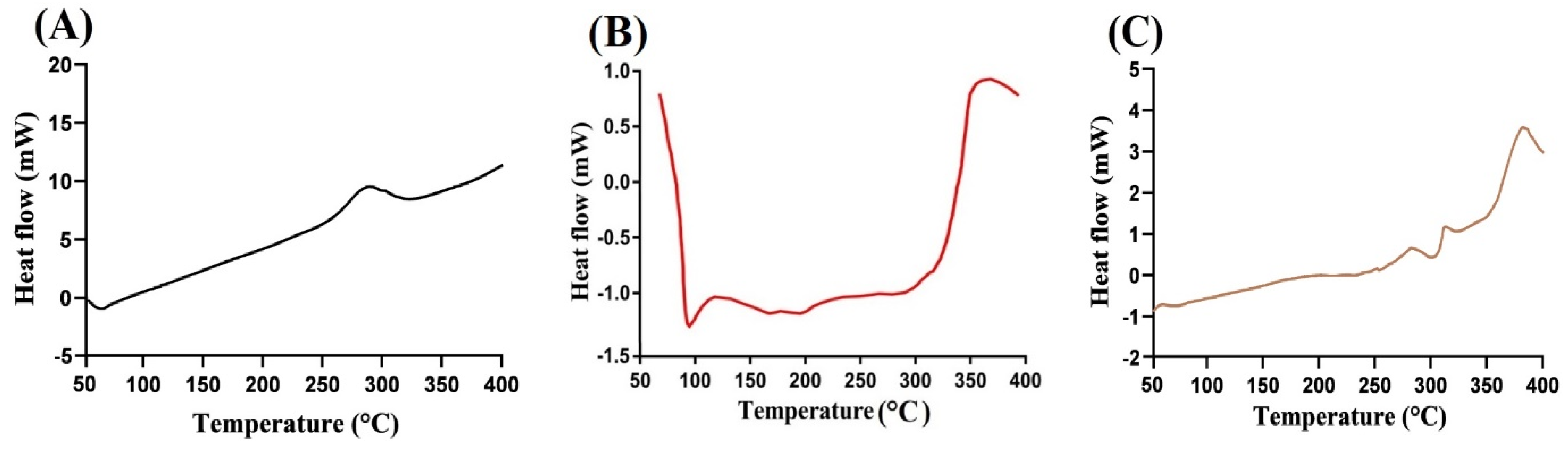
2.3. DSC
2.4. XRD Analysis
2.5. SEM
2.6. Sol–Gel Analysis
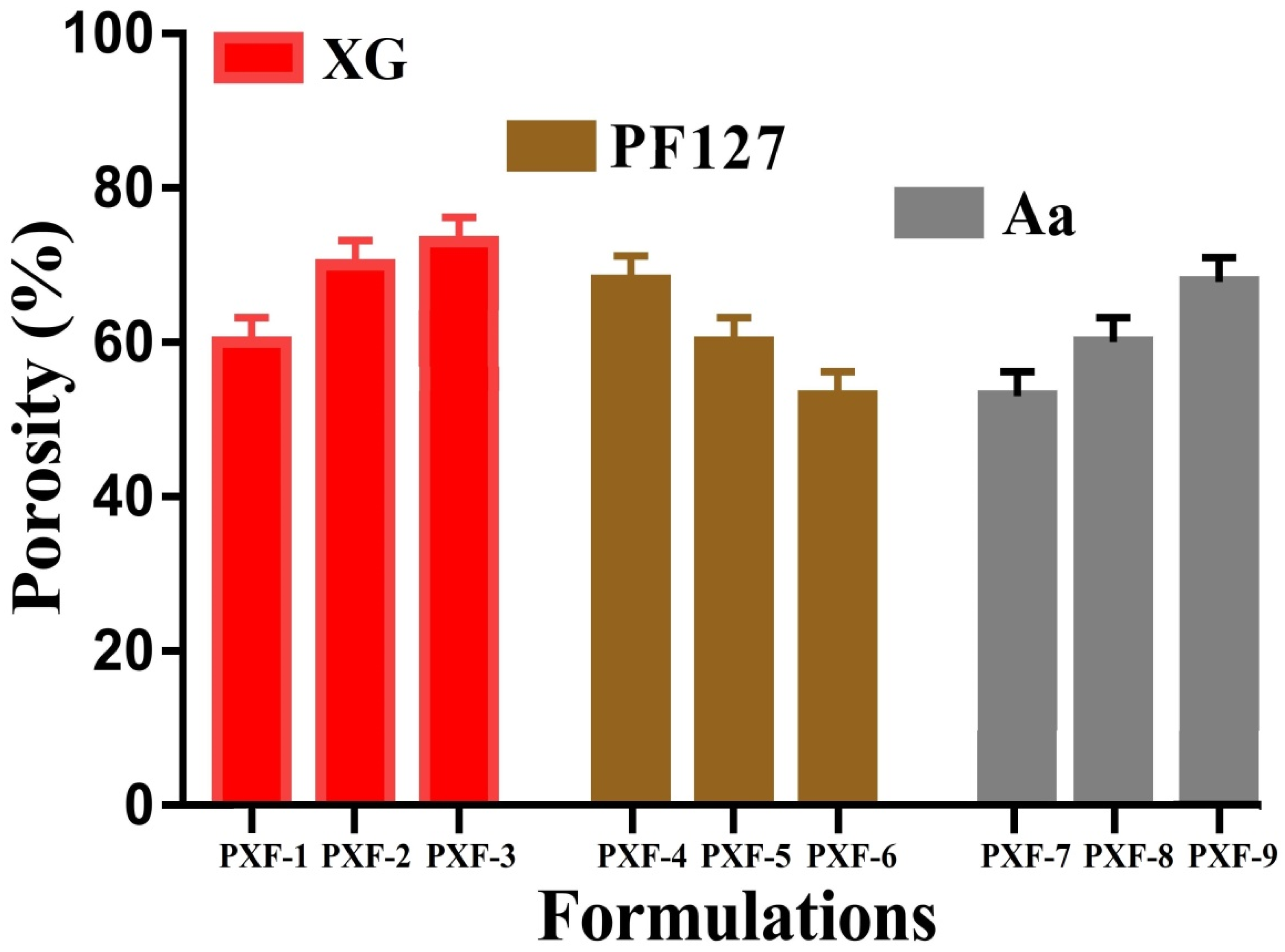
2.7. Porosity
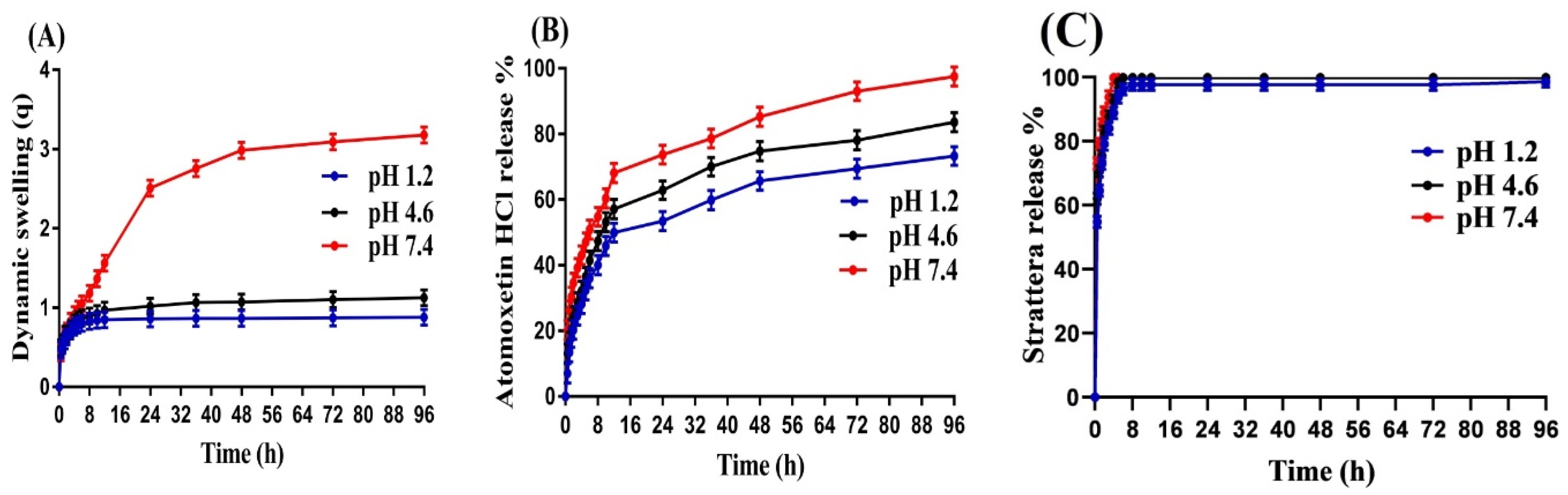
2.8. Swelling Study
2.9. Drug Loading and In Vitro Drug Release Studies
2.10. Release Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods of Preparation
4.3. Characterization
4.4. Sol–Gel Anaylsis
4.5. Porosity
4.6. Swelling Study
4.7. Drug Loading
4.8. In Vitro Drug Release Study
4.9. Release Mechanism
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ayano, G.; Yohannes, K.; Abraha, M. Epidemiology of attention-deficit/hyperactivity disorder (ADHD) in children and adolescents in Africa: A systematic review and meta-analysis. Ann. Gen. Psychiatry 2020, 19, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.; Wright, B.; Partridge, I. Attention deficit hyperactivity disorder—A review. Br. J. Gen. Pract. 1999, 49, 563–571. [Google Scholar] [PubMed]
- De Sousa, A.; Kalra, G. Drug therapy of attention deficit hyperactivity disorder: Current trends. Mens Sana Monogr. 2012, 10, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current concepts and treatments in children and adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef]
- Sevecke, K.; Battel, S.; Dittmann, R.; Lehmkuhl, G.; Döpfner, M. The effectiveness of atomoxetine in children, adolescents, and adults with ADHD. A systematic overview. Der Nervenarzt 2006, 77, 294, 297–300, 302. [Google Scholar] [CrossRef]
- Teaima, M.H.; El-Nadi, M.T.; Hamed, R.R.; El-Nabarawi, M.A.; Abdelmonem, R. Lyophilized Nasal Inserts of Atomoxetine HCl Solid Lipid Nanoparticles for Brain Targeting as a Treatment of Attention-Deficit/Hyperactivity Disorder (ADHD): A Pharmacokinetics Study on Rats. Pharmaceuticals 2023, 16, 326. [Google Scholar] [CrossRef]
- Mohanty, D.; Alsaidan, O.A.; Zafar, A.; Dodle, T.; Gupta, J.K.; Yasir, M.; Mohanty, A.; Khalid, M. Development of Atomoxetine-Loaded NLC In Situ Gel for Nose-to-Brain Delivery: Optimization, In Vitro, and Preclinical Evaluation. Pharmaceutics 2023, 15, 1985. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring atomoxetine release rate from DLP 3D-printed tablets using artificial neural networks: Influence of tablet thickness and drug loading. Molecules 2020, 26, 111. [Google Scholar] [CrossRef]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Alqahtani, M.S.; Mahmood, A.; Barkat, K.; Khan, M.T.; Tulain, U.R.; Rashid, A. β-cyclodextrin chitosan-based hydrogels with tunable pH-responsive properties for controlled release of acyclovir: Design, characterization, safety, and pharmacokinetic evaluation. Drug Deliv. 2021, 28, 1093–1108. [Google Scholar] [CrossRef]
- Pawlicka, A.; Tavares, F.; Dörr, D.; Cholant, C.M.; Ely, F.; Santos, M.; Avellaneda, C. Dielectric behavior and FTIR studies of xanthan gum-based solid polymer electrolytes. Electrochim. Acta 2019, 305, 232–239. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Murtaza, G.; Khalid, Q. Polysaccharide hydrogels for controlled release of acyclovir: Development, characterization and in vitro evaluation studies. Polym. Bull. 2017, 74, 4311–4328. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L.; Piccinini, M.; Marcelli, A. Evaporation-induced crystallization of pluronic F127 studied in situ by time-resolved infrared spectroscopy. J. Phys. Chem. A 2010, 114, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, J.; Li, Y.; Zhao, J.; Dong, H. Engineering of a novel pluronic F127/graphene nanohybrid for pH responsive drug delivery. J. Biomed. Mater. Res. Part A 2012, 100, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Moharram, M.; Khafagi, M. Application of FTIR spectroscopy for structural characterization of ternary poly (acrylic acid)–metal–poly (vinyl pyrrolidone) complexes. J. Appl. Polym. Sci. 2007, 105, 1888–1893. [Google Scholar] [CrossRef]
- Farheen, S.; Zubair, S.; Arpini, A.; Goud, G.; Soppari, S.; Kethavath, M. Formulation and Evaluation of Atomoxetine Hydrochloride Sustained Release Tablets. PharmaTutor 2017, 5, 76–92. [Google Scholar]
- Patil, J.S.; Yadava, S.; Mokale, V.J.; Naik, J.B. Preparation and characterization of single pulse sustained release ketorolac nanoparticles to reduce their side-effects at gastrointestinal tract. In Proceedings of the International Conference on Advances in Chemical Engineering and Technology, Kollam, India, 16–18 October 2014; pp. 59–62. [Google Scholar]
- Jin, J.; Mitome, T.; Egashira, Y.; Nishiyama, N. Phase control of ordered mesoporous carbon synthesized by a soft-templating method. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 58–61. [Google Scholar] [CrossRef]
- Tanaka, S.; Doi, A.; Nakatani, N.; Katayama, Y.; Miyake, Y. Synthesis of ordered mesoporous carbon films, powders, and fibers by direct triblock-copolymer-templating method using an ethanol/water system. Carbon 2009, 47, 2688–2698. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Usman Minhas, M.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly (methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Tummala, S.; Kumar, M.S.; Prakash, A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm. J. 2015, 23, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.; Jana, S.; Sen, K.K. In-vitro release of acyclovir loaded Eudragit RLPO® nanoparticles for sustained drug delivery. Int. J. Biol. Macromol. 2014, 67, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Maiti, S.; Sa, B. Gastrointestinal delivery of glipizide from carboxymethyl locust bean gum–Al3+–alginate hydrogel network: In vitro and in vivo performance. J. Appl. Polym. Sci. 2013, 128, 2063–2072. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, C.-P.; Lee, Y.-D. Synthesis and characterizations of amphiphilic poly (l-lactide)-grafted chondroitin sulfate copolymer and its application as drug carrier. Biomol. Eng. 2007, 24, 131–139. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Zhang, L. Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 2009, 50, 5467–5473. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Preparation and characterization of alginate-PVA-based semi-IPN: Controlled release pH-responsive composites. Polym. Bull. 2018, 75, 1075–1099. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Nam, I.K.; Mun, G.A.; Nurkeeva, Z.S.; Shaikhutdinov, E.M. Radiation synthesis and characterization of stimuli-sensitive chitosan–polyvinyl pyrrolidone hydrogels. Radiat. Phys. Chem. 2005, 72, 619–623. [Google Scholar] [CrossRef]
- Nasir, N.; Ahmad, M.; Minhas, M.U.; Barkat, K.; Khalid, M.F. pH-responsive smart gels of block copolymer [pluronic F127-co-poly (acrylic acid)] for controlled delivery of Ivabradine hydrochloride: Its toxicological evaluation. J. Polym. Res. 2019, 26, 212. [Google Scholar] [CrossRef]
- Yin, L.; Fei, L.; Cui, F.; Tang, C.; Yin, C. Superporous hydrogels containing poly (acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007, 28, 1258–1266. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Qureshi, U.F. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 410. [Google Scholar]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Qudah, Y.; Raafat, A.; Ali, A. Removal of some heavy metals from their aqueous solutions using 2-Acrylamido-2-Methyl-1-propane sulfonic acid/polyvinyl alcohol copolymer hydrogels prepared by gamma irradiation. Arab J. Nucl. Sci. Appl. 2013, 46, 80–91. [Google Scholar]
- Şanlı, O.; Ay, N.; Işıklan, N. Release characteristics of diclofenac sodium from poly (vinyl alcohol)/sodium alginate and poly (vinyl alcohol)-grafted-poly (acrylamide)/sodium alginate blend beads. Eur. J. Pharm. Biopharm. 2007, 65, 204–214. [Google Scholar] [CrossRef]
- Murthy, P.K.; Mohan, Y.M.; Sreeramulu, J.; Raju, K.M. Semi-IPNs of starch and poly (acrylamide-co-sodium methacrylate): Preparation, swelling and diffusion characteristics evaluation. React. Funct. Polym. 2006, 66, 1482–1493. [Google Scholar] [CrossRef]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH-sensitive hydrogels prepared from the blends of poly (vinyl alcohol) with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- Suhail, M.; Hung, M.-C.; Chiu, I.-H.; Vu, Q.L.; Wu, P.-C. Preparation and in-vitro characterization of 5-aminosalicylic acid loaded hydrogels for colon specific delivery. J. Mater. Res. Technol. 2022, 21, 339–352. [Google Scholar] [CrossRef]
- El-Hag Ali, A. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 2012, 123, 763–769. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals 2021, 14, 234. [Google Scholar] [CrossRef]
- Paloma, M.; Enobakhare, Y.; Torrado, G.; Torrado, S. Release of amoxicillin from polyionic complexes of chitosan and poly (acrylic acid). Study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials 2003, 24, 1499–1506. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Li, N.; Gao, J.-Q.; Sun, H.; Chen, S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: Effect of entrapped air. J. Pharm. Sci. 1983, 72, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Wu, P.-C.; Minhas, M.U. Development and characterization of pH-sensitive chondroitin sulfate-co-poly (acrylic acid) hydrogels for controlled release of diclofenac sodium. J. Saudi Chem. Soc. 2021, 25, 101212. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef]
- Zia, M.A.; Sohail, M.; Minhas, M.U.; Sarfraz, R.M.; Khan, S.; de Matas, M.; Hussain, Z.; Abbasi, M.; Shah, S.A.; Kousar, M. HEMA based pH-sensitive semi IPN microgels for oral delivery; a rationale approach for ketoprofen. Drug Dev. Ind. Pharm. 2020, 46, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, H.; Tulain, U.R.; Azam, F.; Qureshi, J. Thiolation of arabinoxylan and its application in the fabrication of pH-sensitive thiolated arabinoxylan grafted acrylic acid copolymer. Drug Dev. Ind. Pharm. 2019, 45, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I.; Malik, N.S. Chondroitin sulfate-based smart hydrogels for targeted delivery of oxaliplatin in colorectal cancer: Preparation, characterization and toxicity evaluation. Polym. Bull. 2020, 77, 6271–6297. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]



| Formulation Code | Sol Fraction (%) | Gel Fraction (%) | Drug Loaded (mg)/350 mg of Dry Gel | |
|---|---|---|---|---|
| Weight Method | Extraction Method | |||
| PXF-1 | 11 | 89 | 132.10 ± 0.71 | 130.84 ± 1.03 |
| PXF-2 | 9 | 91 | 155.23 ± 0.93 | 152.42 ± 0.64 |
| PXF-3 | 7 | 93 | 164.01 ± 1.10 | 163.02 ± 0.85 |
| PXF-4 | 15 | 85 | 102.34 ± 0.78 | 101.04 ± 0.94 |
| PXF-5 | 12 | 88 | 93.61 ± 0.93 | 91.23 ± 1.01 |
| PXF-6 | 10 | 90 | 86.03 ± 1.03 | 84.87 ± 0.84 |
| PXF-7 | 16 | 84 | 143.44 ± 0.87 | 142.31 ± 0.92 |
| PXF-8 | 14 | 86 | 161.05 ± 0.91 | 159.92 ± 0.48 |
| PXF-9 | 13 | 87 | 170.72 ± 0.83 | 168.63 ± 1.20 |
| F. Code | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | N | |
| PXF-1 | 0.9432 | 0.9920 | 0.8937 | 0.9582 | 0.5331 |
| PXF-2 | 0.9356 | 0.9978 | 0.9220 | 0.9819 | 0.5562 |
| PXF-3 | 0.9682 | 0.9870 | 0.9712 | 0.9754 | 0.5040 |
| PXF-4 | 0.9548 | 0.9764 | 0.9692 | 0.9625 | 0.5128 |
| PXF-5 | 0.9793 | 0.9954 | 0.9830 | 0.9922 | 0.5673 |
| PXF-6 | 0.9450 | 0.9788 | 0.9706 | 0.9414 | 0.5468 |
| PXF-7 | 0.9890 | 0.9903 | 0.9790 | 0.9378 | 0.5219 |
| PXF-8 | 0.9063 | 0.9661 | 0.9332 | 0.9627 | 0.5493 |
| PXF-9 | 0.9274 | 0.9845 | 0.9568 | 0.9439 | 0.5790 |
| F. Code | Polymer (XG) g/30 g | Polymer (PF-127) g/30 g | Monomer (Aa) g/30 g | Initiator (APS) g/30 g | Cross-linker (MBA) g/30 g |
|---|---|---|---|---|---|
| PXF-1 | 0.080 | 0.200 | 4.0 | 0.1 | 0.2 |
| PXF-2 | 0.120 | 0.200 | 4.0 | 0.1 | 0.2 |
| PXF-3 | 0.160 | 0.200 | 4.0 | 0.1 | 0.2 |
| PXF-4 | 0.050 | 0.300 | 4.0 | 0.1 | 0.2 |
| PXF-5 | 0.050 | 0.350 | 4.0 | 0.1 | 0.2 |
| PXF-6 | 0.050 | 0.400 | 4.0 | 0.1 | 0.2 |
| PXF-7 | 0.050 | 0.200 | 4.5 | 0.1 | 0.2 |
| PXF-8 | 0.050 | 0.200 | 5.0 | 0.1 | 0.2 |
| PXF-9 | 0.050 | 0.200 | 5.5 | 0.1 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhail, M.; Chiu, I.-H.; Lai, Y.-R.; Khan, A.; Al-Sowayan, N.S.; Ullah, H.; Wu, P.-C. Xanthan-Gum/Pluronic-F-127-Based-Drug-Loaded Polymeric Hydrogels Synthesized by Free Radical Polymerization Technique for Management of Attention-Deficit/Hyperactivity Disorder. Gels 2023, 9, 640. https://doi.org/10.3390/gels9080640
Suhail M, Chiu I-H, Lai Y-R, Khan A, Al-Sowayan NS, Ullah H, Wu P-C. Xanthan-Gum/Pluronic-F-127-Based-Drug-Loaded Polymeric Hydrogels Synthesized by Free Radical Polymerization Technique for Management of Attention-Deficit/Hyperactivity Disorder. Gels. 2023; 9(8):640. https://doi.org/10.3390/gels9080640
Chicago/Turabian StyleSuhail, Muhammad, I-Hui Chiu, Yi-Ru Lai, Arshad Khan, Noorah Saleh Al-Sowayan, Hamid Ullah, and Pao-Chu Wu. 2023. "Xanthan-Gum/Pluronic-F-127-Based-Drug-Loaded Polymeric Hydrogels Synthesized by Free Radical Polymerization Technique for Management of Attention-Deficit/Hyperactivity Disorder" Gels 9, no. 8: 640. https://doi.org/10.3390/gels9080640






