The Development of Fe3O4-Monolithic Resorcinol-Formaldehyde Carbon Xerogels Using Ultrasonic-Assisted Synthesis for Arsenic Removal of Drinking Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fe3O4-Monolithic Resorcinol-Formaldehyde Xerogels: Effect of Loading of Magnetite with Indirect and Direct Sonication, and Modification of Catalyst
2.1.1. Characterization of MCs and MXs
2.1.2. Performance of Adsorption of Arsenic Using MCs and MXs
2.2. Fe3O4-Monolithic Resorcinol-Formaldehyde Xerogels with Direct and Indirect Sonications: Effects of Power Output of Ultrasonic Processor, Varying the Molar Ratios of M/R and R/W
2.2.1. Characterization of Fe3O4-Monolithic Resorcinol-Formaldehyde Xerogels MX3-MX7 and MX8-MX11
2.2.2. Performance of Adsorption of Arsenic Using MX4-MX7 and MX8-MX11
2.3. Fe3O4-Monolithic Resorcinol-Formaldehyde Xerogels and Carbon Xerogels by Indirect Sonication
2.3.1. Characterization of Fe3O4-Monolithic Resorcinol-Formaldehyde Carbon Xerogels
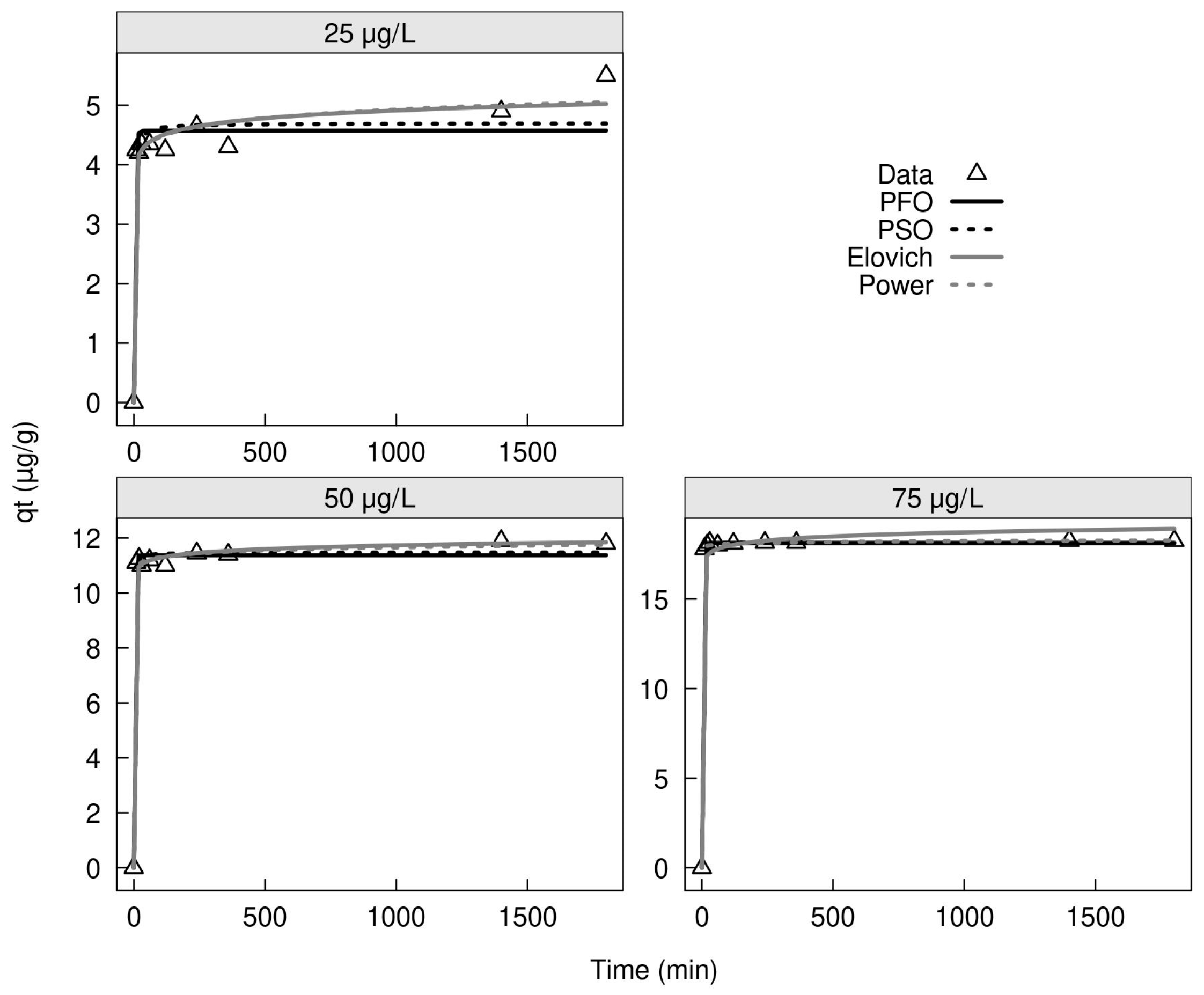
2.3.2. Adsorption of Low and High Concentration of As(III) and As(V) with MXRF and MXRF600
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis of Adsorbent Materials
4.2.1. Monolithic Resorcinol-Formaldehyde Xerogels Effect of Loading of Magnetite with Direct and Indirect Sonication, and Modification of Catalyst
4.2.2. Monolithic Resorcinol-Formaldehyde Xerogels with Direct and Indirect Sonication: Effects of Power Output of Ultrasonic Processor, Varying the Molar Ratios of M/R and R/W
4.2.3. Monolithic Resorcinol-Formaldehyde Carbon Xerogels by Indirect Sonication
4.3. Characterization through Analytical Techniques
4.4. Batch Adsorption Experiment
4.5. Determination of As(III) and As(V)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Arsenic. Atlanta 2007, 1–559. [Google Scholar]
- Environmental Protection Agency (EPA) National Primary Drinking Water Regulations: Long Term 1 Enhanced Surface Water Treatment Rule. Fed. Regist. 2002, 75, 1812–1844.
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Secretary of Health (SSA). Official Mexican Standards NOM-127-SSA1-2021, Environmental Health, Water for Human Use and Consumption—Permissible Limits of Quality and Treatments to Which Water Must Be Submitted for Its Drinkability; Official Daily of the Federation: Mexico City, Mexico, 2021; pp. 1–121. [Google Scholar]
- Pal, P.; Sen, M.; Manna, A.; Pal, J.; Pal, P.; Roy, S.; Roy, P. Contamination of groundwater by arsenic: A review of occurrence, causes, impacts, remedies and membrane-based purification. J. Integr. Environ. Sci. 2009, 6, 295–316. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Shanker, U. Shikha Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.H.; Jiménez-Córdova, M.I.; Cárdenas-González, M.; Sánchez Retana, I.M.; Gonsebatt, M.E.; Del Razo, L.M. Potential co-exposure to arsenic and fluoride and biomonitoring equivalents for Mexican children. Ann. Glob. Health 2018, 84, 257–273. [Google Scholar] [CrossRef] [Green Version]
- González-Horta, C.; Ballinas-Casarrubias, L.; Sánchez-Ramírez, B.; Ishida, M.C.; Barrera-Hernández, A.; Gutiérrez-Torres, D.; Zacarias, O.L.; Jesse Saunders, R.; Drobná, Z.; Mendez, M.A.; et al. A concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2015, 12, 4587–4601. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, R.; Morales-Arredondo, I.; Rodríguez, I. Geological differentiation of groundwater threshold concentrations of arsenic, vanadium and fluorine in El Bajio Guanajuatense, Mexico. Geofis. Int. 2016, 55, 5–15. [Google Scholar] [CrossRef]
- Vega-Millán, C.B.; Dévora-Figueroa, A.G.; Burgess, J.L.; Beamer, P.I.; Furlong, M.; Lantz, R.C.; Meza-Figueroa, D.; O’Rourke, M.K.; García-Rico, L.; Meza-Escalante, E.R.; et al. Inflammation biomarkers associated with arsenic exposure by drinking water and respiratory outcomes in indigenous children from three Yaqui villages in southern Sonora, México. Environ. Sci. Pollut. Res. 2021, 28, 34355–34366. [Google Scholar] [CrossRef]
- Martínez-Acuña, M.I.; Mercado-Reyes, M.; Alegría-Torres, J.A.; Mejía-Saavedra, J.J. Preliminary human health risk assessment of arsenic and fluoride in tap water from Zacatecas, México. Environ. Monit. Assess. 2016, 188, 476. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; Van Der Kuijp, T.J. The health effects of exposure to arsenic-contaminated drinking water: A review by global geographical distribution. Int. J. Environ. Health Res. 2015, 25, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Monrad, M.; Ersbøll, A.K.; Sørensen, M.; Baastrup, R.; Hansen, B.; Gammelmark, A.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017, 154, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Loira, B.; Cebrián, M.E.; López-Carrillo, L. Arsenic exposure in northern Mexican women. Salud Publica Mex. 2020, 62, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Scannell Bryan, M.; Argos, M. Association between prenatal arsenic exposure, birth outcomes, and pregnancy complications: An observational study within the National Children’s Study cohort. Environ. Res. 2020, 183, 109182. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Wang, X.; Wu, Y.; Zhang, Y.; Chen, S.; Zhang, W.; Sun, X.; Zheng, T.; Xia, W.; et al. Prenatal arsenic exposure, arsenic metabolism and neurocognitive development of 2-year-old children in low-arsenic areas. Environ. Int. 2023, 174, 107918. [Google Scholar] [CrossRef]
- Bibi, S.; Kamran, M.A.; Sultana, J.; Farooqi, A. Occurrence and methods to remove arsenic and fluoride contamination in water. Environ. Chem. Lett. 2017, 15, 125–149. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Amiri, S.; Vatanpour, V.; He, T. Optimization of Coagulation-Flocculation Process in Efficient Arsenic Removal from Highly Contaminated Groundwater by Response Surface Methodology. Molecules 2022, 27, 7953. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; El Ibrahimi, B.; Abou Oualid, H.; Kassab, Z.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Gamero-Melo, P. Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As(V) ions: Experimental and theoretical studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Wang, H.; Qi, X.; Yan, G.; Shi, J. Copper-doped ZIF-8 nanomaterials as an adsorbent for the efficient removal of As(V) from wastewater. J. Phys. Chem. Solids 2023, 179, 111408. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.S.; Choi, Y.L.; Chang, Y.Y.; Koduru, J.R. Stable and recyclable lanthanum hydroxide–doped graphene oxide biopolymer foam for superior aqueous arsenate removal: Insight mechanisms, batch, and column studies. Chemosphere 2023, 313, 137615. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, Y.; Huang, C.; Liu, Q. Amine functionalization of iron-based metal-organic frameworks MIL-101 for removal of arsenic species: Enhanced adsorption and mechanisms. J. Environ. Chem. Eng. 2023, 11, 110155. [Google Scholar] [CrossRef]
- López-Martínez, A.M.; Khamkure, S.; Gamero-Melo, P. Bifunctional Adsorbents Based on Jarosites for Removal of Inorganic Micropollutants from Water. Separations 2023, 10, 309. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.V.; Vigneswaran, S.; Ha, N.T.H.; Ratnaweera, H. A Review of Theoretical Knowledge and Practical Applications of Iron-Based Adsorbents for Removing Arsenic from Water. Minerals 2023, 13, 741. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Mînzatu, V.; Davidescu, C.M.; Negrea, P.; Ciopec, M.; Muntean, C.; Hulka, I.; Paul, C.; Negrea, A.; Duțeanu, N. Synthesis, characterization and adsorptive performances of a composite material based on carbon and iron oxide particles. Int. J. Mol. Sci. 2019, 20, 1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierro, V.; Celzard, A.; Fierro, V.; Szczurek, A.; Braghiroli, F.; Parmentier, J.; Pizzi, A.; Suhfxuvruv, S.; Fduerq, R.I.; Dqg, D.; et al. Carbon gels derived from natural resources Geles de carbón de origen natural. 2012, 1–7. Available online: https://digital.csic.es/handle/10261/81796 (accessed on 19 April 2023).
- Huang, G.; Li, W.; Song, Y. Preparation of SiO2–ZrO2 xerogel and its application for the removal of organic dye. J. Sol-Gel Sci. Technol. 2018, 86, 175–186. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Regufe, M.J.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Resorcinol–formaldehyde carbon xerogel as selective adsorbent of carbon dioxide present on biogas. Adsorption 2018, 24, 169–177. [Google Scholar] [CrossRef]
- Wickenheisser, M.; Herbst, A.; Tannert, R.; Milow, B.; Janiak, C. Hierarchical MOF-xerogel monolith composites from embedding MIL-100(Fe, Cr) and MIL-101(Cr) in resorcinol-formaldehyde xerogels for water adsorption applications. Microporous Mesoporous Mater. 2015, 215, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Prasad, S.; Saxena, P.N.; Ansari, N.G.; Patel, D.K. Synthesis of an Alginate-Based Fe3O4-MnO2Xerogel and Its Application for the Concurrent Elimination of Cr(VI) and Cd(II) from Aqueous Solution. ACS Omega 2021, 6, 3931–3945. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanoparticle Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennas, G.; Gedanken, A.; Mannias, G.; Kumar, V.B.; Scano, A.; Porat, Z.; Pilloni, M. Formation of Iron (III) Trimesate Xerogel by Ultrasonic Irradiation. Eur. J. Inorg. Chem. 2022, 2022, e202101082. [Google Scholar] [CrossRef]
- Maeda, Y.; Hayashi, Y.; Fukushima, J.; Takizawa, H. Sonochemical effect and pore structure tuning of silica xerogel by ultrasonic irradiation of semi-solid hydrogel. Ultrason. Sonochem. 2021, 73, 105476. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hauschild, M.Z. Indicators for Environmental Sustainability. Procedia CIRP 2017, 61, 697–702. [Google Scholar] [CrossRef]
- Oyedoh, E.A.; Albadarin, A.B.; Walker, G.M.; Mirzaeian, M.; Ahmad, M.N.M. Preparation of controlled porosity resorcinol formaldehyde xerogels for adsorption applications. Chem. Eng. Trans. 2013, 32, 1651–1656. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Menéndez, J.A.; Arenillas, A. Carbon Xerogels: The Bespoke Nanoporous Carbons; Ghrib, T.H., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 3; ISBN 978-1-78923-043-7. [Google Scholar]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Sulistyaningsih, T.; Santosa, S.J.; Siswanta, D.; Rusdiarso, B. Synthesis and Characterization of Magnetites Obtained from Mechanically and Sonochemically Assissted Co-precipitation and Reverse Co-precipitation Methods. Int. J. Mater. Mech. Manuf. 2017, 5, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, M.M.; Sarode, K.K.; Pathak, A.D.; Sharma, C.S. Ultrahigh rate and high-performance lithium-sulfur batteries with resorcinol-formaldehyde xerogel derived highly porous carbon matrix as sulfur cathode host. Chem. Eng. J. 2021, 425, 131521. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Ros, C.H.; Morales-Torres, S.; Pérez-Cadenas, M.; Kooyman, P.J.; Moreno-Castilla, C.; Kapteijn, F. Metal-doped carbon xerogels for the electro-catalytic conversion of CO2to hydrocarbons. Carbon N. Y. 2013, 56, 324–331. [Google Scholar] [CrossRef]
- Prostredný, M.; Abduljalil, M.; Mulheran, P.; Fletcher, A. Process Variable Optimization in the Manufacture of Resorcinol–Formaldehyde Gel Materials. Gels 2018, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, E.; Prostredny, M.; Fletcher, A.; Mulheran, P. Modelling organic gel growth in three dimensions: Textural and fractal properties of resorcinol–formaldehyde gels. Gels 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Khare, P.; Verma, N. Synthesis of iron-doped resorcinol formaldehyde-based aerogels for the removal of Cr(VI) from water. Green Process. Synth. 2015, 4, 37–46. [Google Scholar] [CrossRef]
- Sharifalhoseini, Z.; Entezari, M.H.; Jalal, R. Direct and indirect sonication affect differently the microstructure and the morphology of ZnO nanoparticles: Optical behavior and its antibacterial activity. Ultrason. Sonochem. 2015, 27, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.G.; Menéndez, J.Á.; Arenillas, A. Designing Nanostructured Carbon Xerogels. In Nanomaterials; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef] [Green Version]
- Job, N.; Panariello, F.; Marien, J.; Crine, M.; Pirard, J.P.; Léonard, A. Synthesis optimization of organic xerogels produced from convective air-drying of resorcinol-formaldehyde gels. J. Non. Cryst. Solids 2006, 352, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Mirzaeian, M.; Hall, P.J. The control of porosity at nano scale in resorcinol formaldehyde carbon aerogels. J. Mater. Sci. 2009, 44, 2705–2713. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Prostredny, M.; Fletcher, A. Investigating the role of the catalyst within resorcinol– formaldehyde gel synthesis. Gels 2021, 7, 142. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Calvo, E.G.; Menéndez, J.A.; Arenillas, A. Influence of alkaline compounds on the porosity of resorcinol-formaldehyde xerogels. J. Non. Cryst. Solids 2016, 452, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Luzny, R.; Ignasiak, M.; Walendziewski, J.; Stolarski, M. Heavy metal ions removal from aqueous solutions using carbon aerogels and xerogels. Chemik 2014, 68, 544–553. [Google Scholar]
- Alonso-Buenaposada, I.D.; Montes-Morán, M.A.; Menéndez, J.A.; Arenillas, A. Synthesis of hydrophobic resorcinol–formaldehyde xerogels by grafting with silanes. React. Funct. Polym. 2017, 120, 92–97. [Google Scholar] [CrossRef]
- Dewes, R.M.; Mendoza, H.R.; Pereira, M.V.L.; Lutz, C.; Gerven, T. Van Experimental and numerical investigation of the effect of ultrasound on the growth kinetics of zeolite A. Ultrason. Sonochem. 2022, 82, 105909. [Google Scholar] [CrossRef]
- Ferri, S.; Wu, Q.; De Grazia, A.; Polydorou, A.; May, J.P.; Stride, E.; Evans, N.D.; Carugo, D. Tailoring the size of ultrasound responsive lipid-shelled nanodroplets by varying production parameters and environmental conditions. Ultrason. Sonochem. 2021, 73, 105482. [Google Scholar] [CrossRef] [PubMed]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.P. Porous carbon xerogels with texture tailored by pH control during sol-gel process. Carbon N. Y. 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.; Sharma, A.K.; Hong, W.G.; Shin, K.; Song, H.; Kim, H.Y.; Hong, Y.J. Recyclable Aqueous Metal Adsorbent: Synthesis and Cu(II) Sorption Characteristics of Ternary Nanocomposites of Fe3O4 Nanoparticles@Graphene–poly-N-Phenylglycine Nanofibers. J. Hazard. Mater. 2021, 401, 123283. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Abdelfatah, M.S.; Mossad, M.M. Conduction mechanism and dielectric properties of pure and composite resorcinol formaldehyde aerogels doped with silver. J. Phys. Conf. Ser. 2017, 869, 012035. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Rey-Raap, N.; Menéndez, J.Á.; Montes-Morán, M.A.; Figueiredo, J.L.; Pereira, M.F.R.; Arenillas, A. Effect of porous structure on doping and the catalytic performance of carbon xerogels towards the oxygen reduction reaction. Microporous Mesoporous Mater. 2020, 293, 109811. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Yang, Y.; Mi, J.H.; Tan, X.L. Synthesis, characterisation and magnetic examination of Fe, Co and Ni doped carbon xerogels. Mater. Res. Innov. 2012, 16, 362–367. [Google Scholar] [CrossRef]
- Alegre, C.; Sebastián, D.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Aricò, A.S.; Baglio, V.; Lázaro, M.J. N-Doped Carbon Xerogels as Pt Support for the Electro-Reduction of Oxygen. Materials 2017, 10, 1092. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Fernández, G.; Canal-Rodríguez, M.; Arenillas, A.; Menéndez, J.A.; Rodríguez-Pastor, I.; Martin-Gullon, I. Determinant influence of the electrical conductivity versus surface area on the performance of graphene oxide-doped carbon xerogel supercapacitors. Carbon N. Y. 2018, 126, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, A.; Wang, J.; Khan, M.S.; Farooq, U.; Riaz, N.; Nazir, A.; Mahmood, Q.; Hashem, A.; Al-Arjani, A.B.F.; Alqarawi, A.A.; et al. Iron oxide (Fe3O4)-supported sio2 magnetic nanocomposites for efficient adsorption of fluoride from drinking water: Synthesis, characterization, and adsorption isotherm analysis. Water 2021, 13, 1514. [Google Scholar] [CrossRef]
- Nikić, J.; Tubić, A.; Watson, M.; Maletić, S.; Šolić, M.; Majkić, T.; Agbaba, J. Arsenic removal from water by green synthesized magnetic nanoparticles. Water 2019, 11, 2520. [Google Scholar] [CrossRef] [Green Version]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Haro, M.; Rasines, G.; MacIas, C.; Ania, C.O. Stability of a carbon gel electrode when used for the electro-assisted removal of ions from brackish water. Carbon N. Y. 2011, 49, 3723–3730. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Amaringo, F.A.; Anaguano, A. Determination of the point of zero charge and isoelectric point of two agricultural wastes and their application in the removal of colorants. Rev. Investig. Agrar. y Ambient. 2013, 4, 27. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.d.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.d.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 950. [Google Scholar] [CrossRef] [Green Version]
- Hanbali, M.; Holail, H.; Hammud, H. Remediation of lead by pretreated red algae: Adsorption isotherm, kinetic, column modeling and simulation studies. Green Chem. Lett. Rev. 2014, 7, 342–358. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Quevedo, O.; Luna, B. Determinación de As (III) y As (V) en aguas naturales por generación de hidruro con detección por espectrometría de absorción atómica. Rev. CENIC. Ciencias Químicas 2003, 34, 133–147. [Google Scholar]
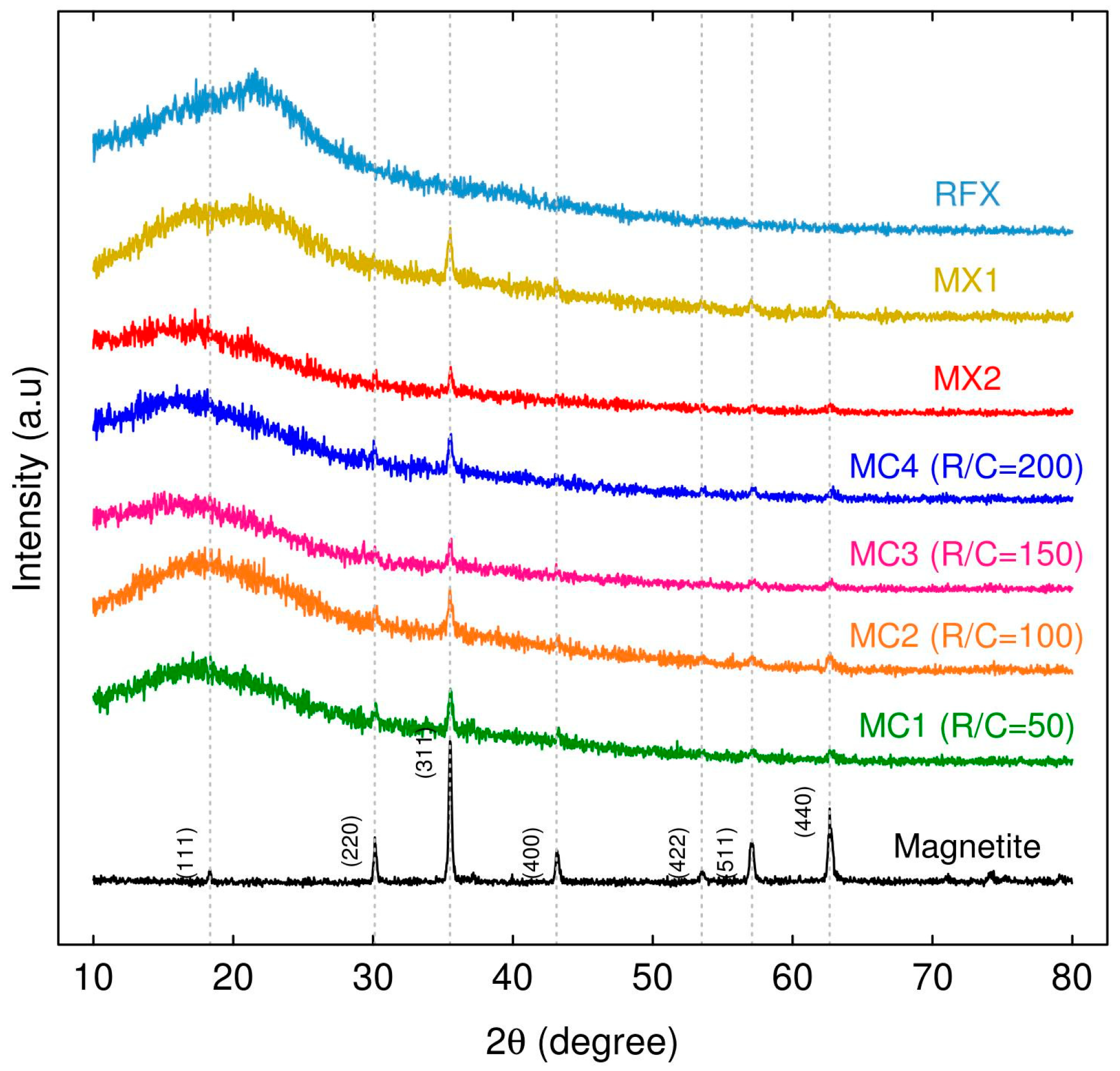
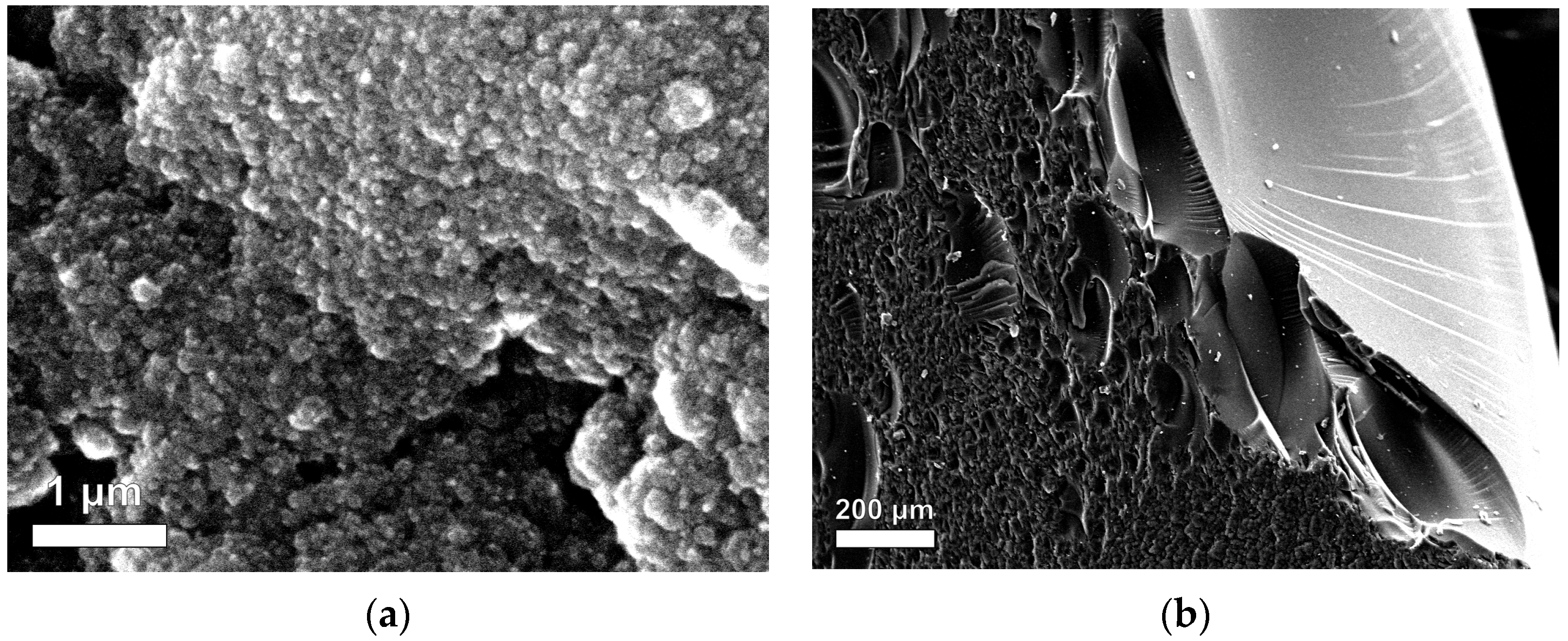
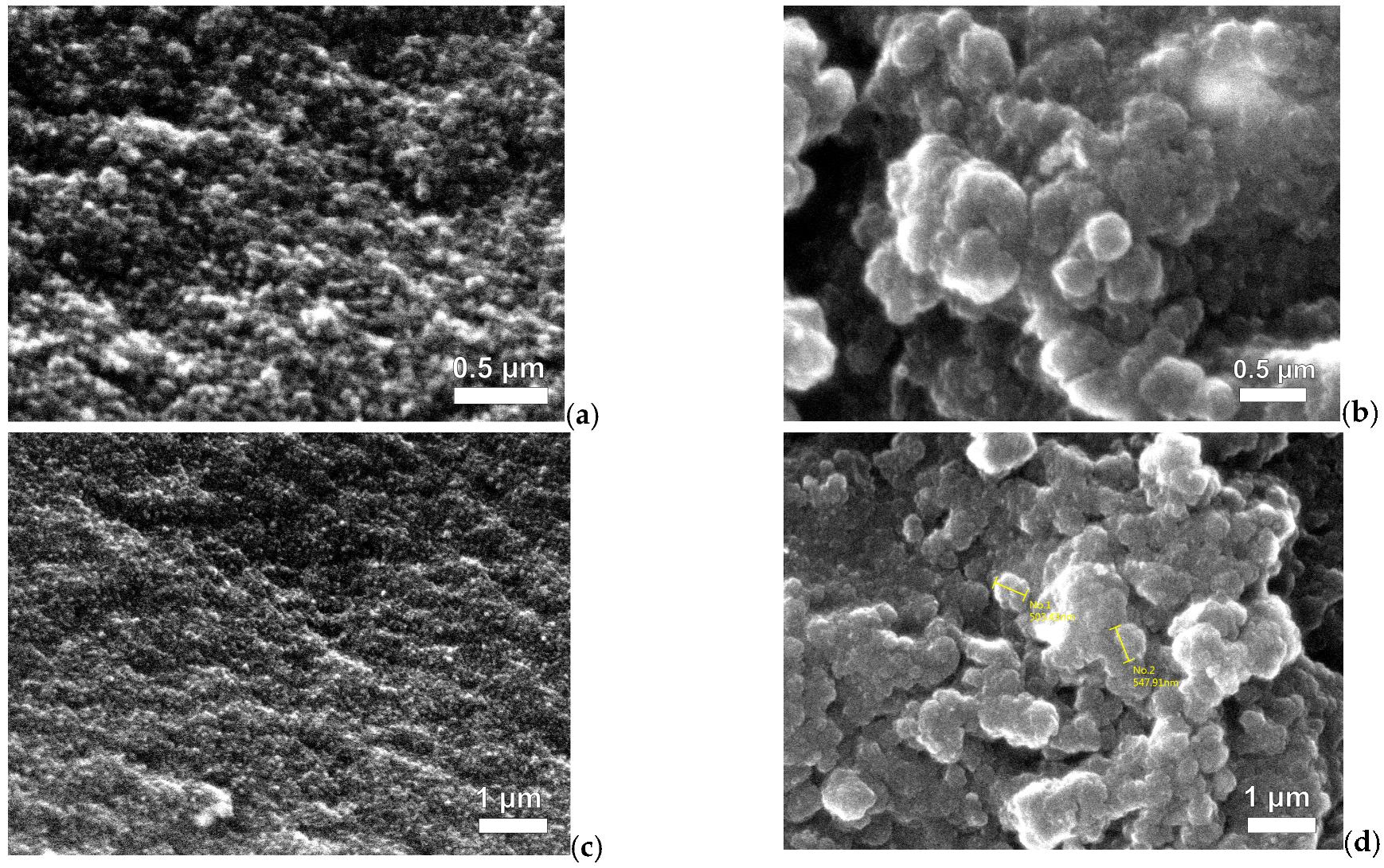
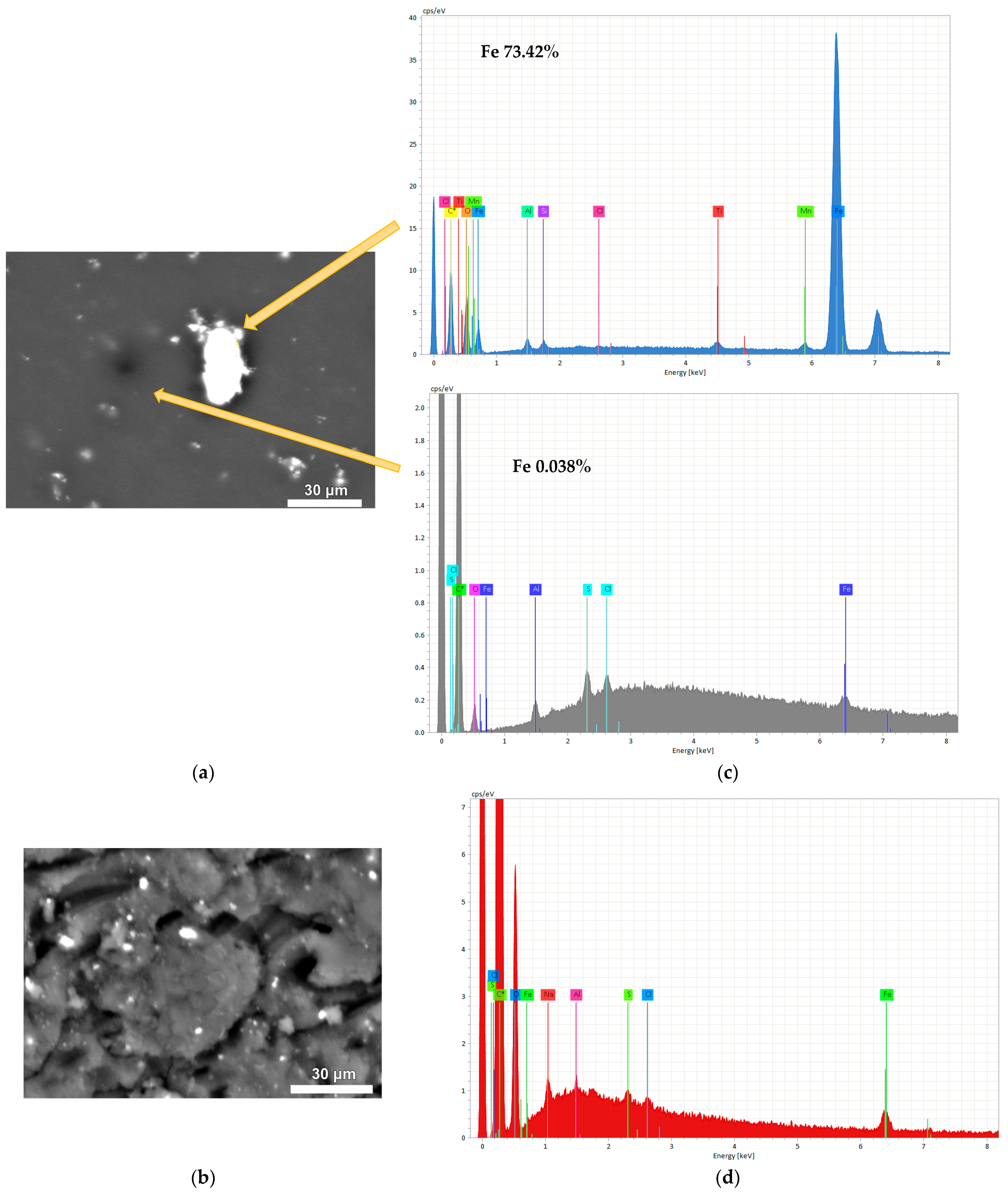

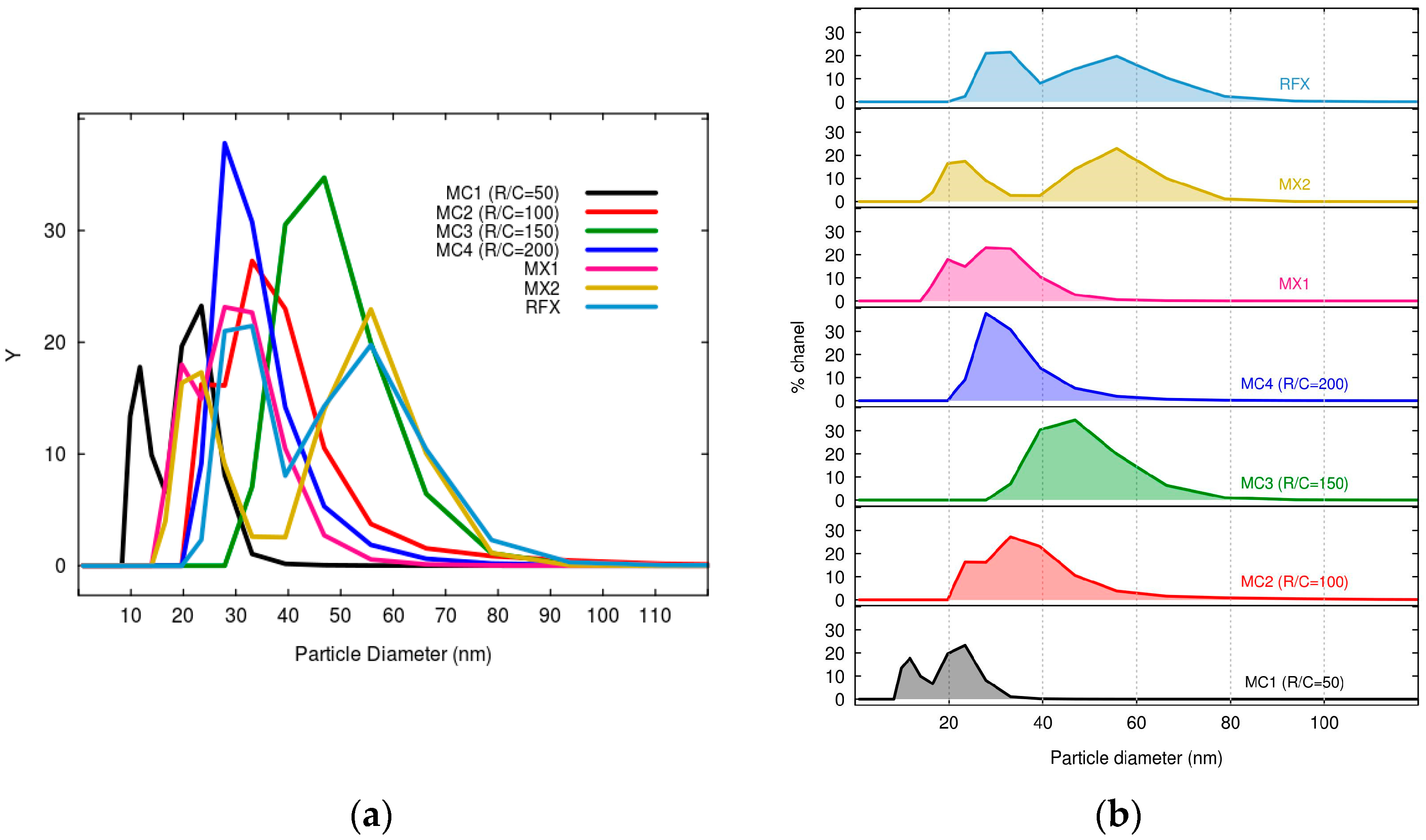





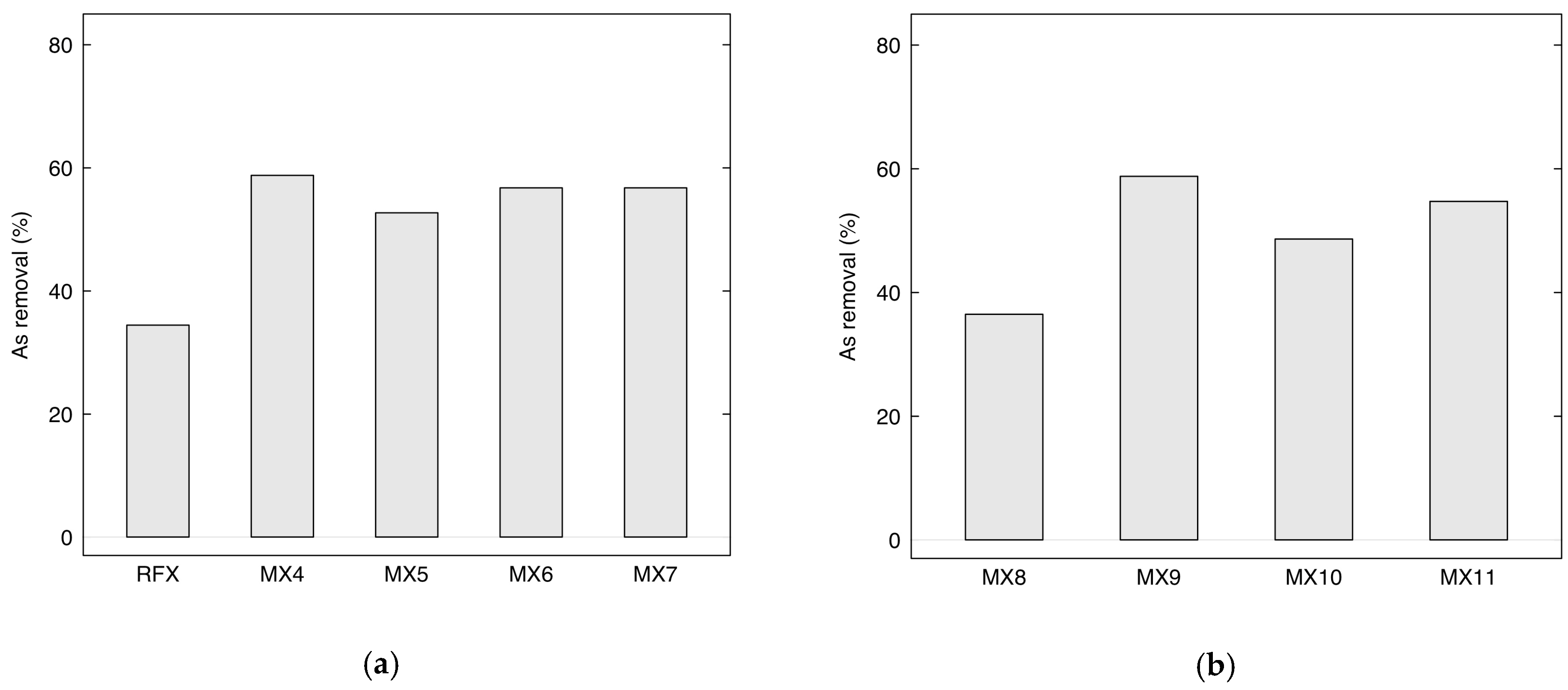
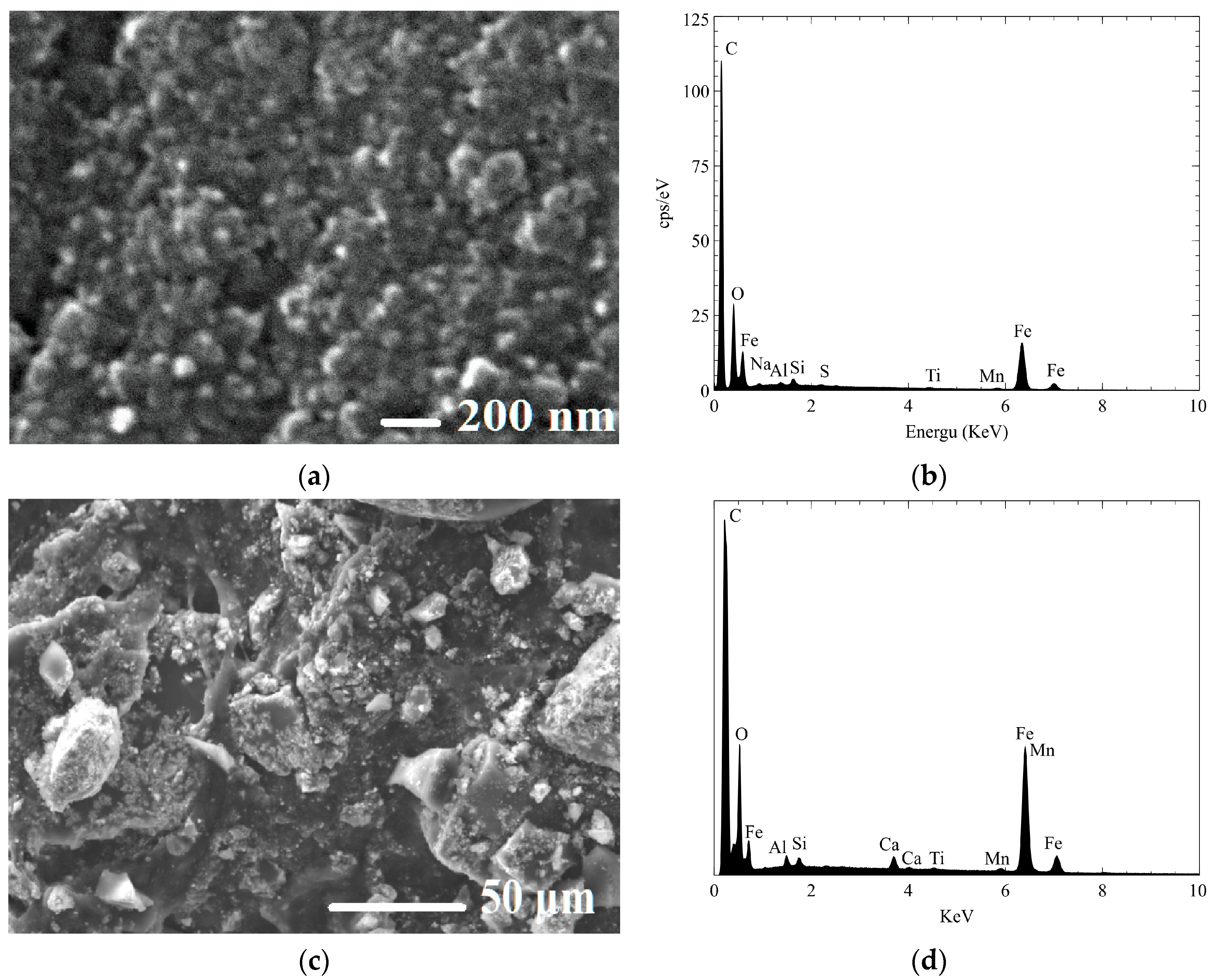

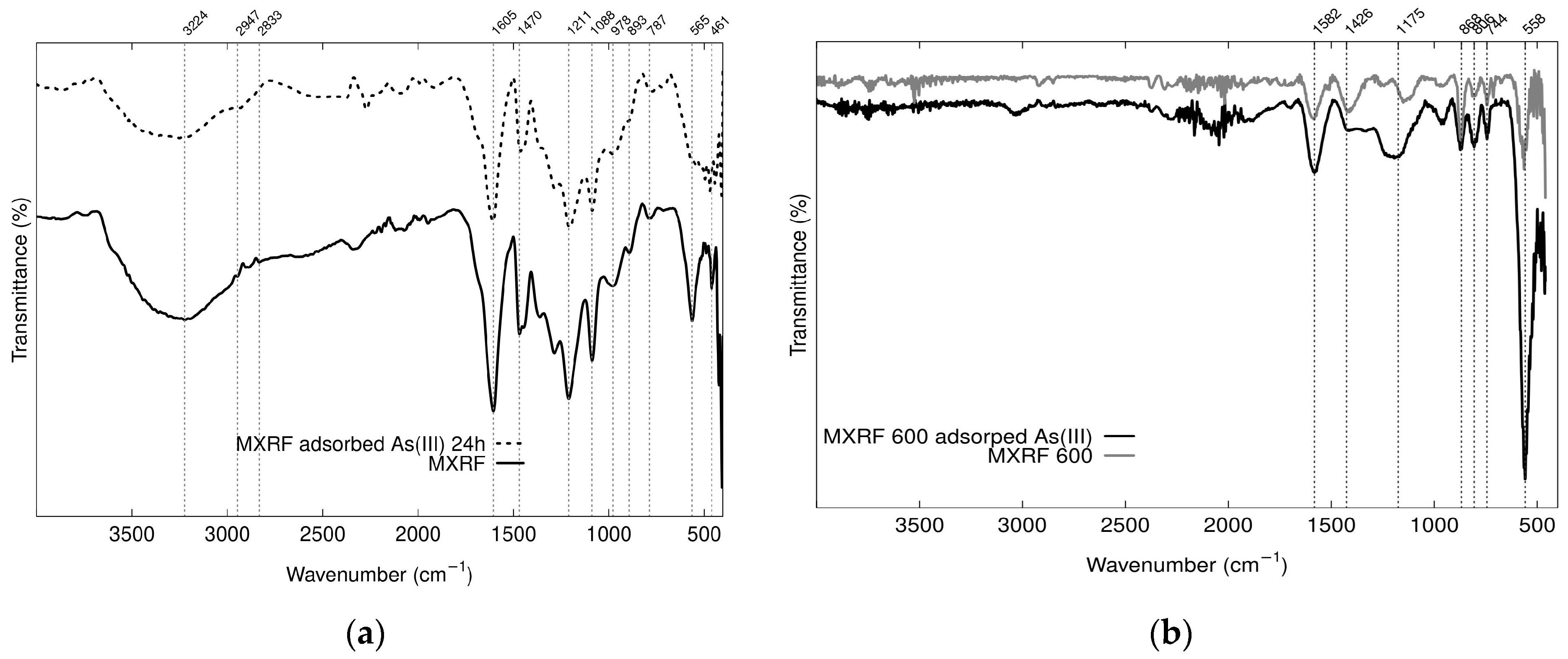

| Sample Name | R/C Molar Ratios | Crystal Size Average (nm) | FWHM (β) (Radian) | θ (Radian) |
|---|---|---|---|---|
| MC1 | 50 | 24.52 | 0.34 | 35.54 |
| MC2 | 100 | 22.94 | 0.36 | 35.52 |
| MC4 | 200 | 25.88 | 0.32 | 35.53 |
| Samples | Molar Ratio of R/C | Area BET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | pHpzc | IEP |
|---|---|---|---|---|---|---|
| RFX | 200 | 399.19 | 0.517 | 5.23 | 2.99 | 2.74 |
| MX1 | 200 | 472.41 | 0.842 | 7.57 | 4.54 | 3.09 |
| MC1 | 50 | 365.93 | 0.255 | 2.79 | 6.63 | 3.40 |
| MC2 | 100 | 545.09 | 0.549 | 4.03 | 6.12 | 3.59 |
| MC4 | 200 | 529.47 | 0.683 | 5.16 | 4.35 | 3.70 |
| Molar Ratios | Direct Ultrasonic-Assisted Synthesis | ||||
|---|---|---|---|---|---|
| MX3 | MX4 | MX5 | MX6 | MX7 | |
| R/W | 0.04 | ||||
| R/C | 200 | ||||
| R/F | 0.5 | ||||
| M/R | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 |
| Fe content (w%) | 3.48 | 5.62 | 9.29 | 13.39 | 13.13 |
| Solids content (w/v%) | 19.59 | 20.37 | 22.29 | 24.23 | 26.16 |
| Molar Ratios | Indirect Ultrasonic-Assisted Synthesis | |||
|---|---|---|---|---|
| MX8 | MX9 | MX10 | MX11 | |
| R/W | 0.05 | |||
| R/C | 200 | |||
| R/F | 0.5 | |||
| M/R | 0.03 | 0.05 | 0.1 | 0.15 |
| Fe content (w%) | 3.41 | 5.82 | 11.59 | 16.09 |
| Solids content (w/v%) | 23.04 | 23.95 | 26.22 | 28.48 |
| Adsorption | Pseudo First-Order | Pseudo Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|
| qt (µg/g) | k1 | R² | RMSE | qt (µg/g) | k2 | R² | RMSE | |
| As(III) | 129.68 | 0.153 | 0.374 | 9.900 | 134.03 | 0.002 | 0.575 | 8.161 |
| As(V) | 230.55 | 0.147 | 0.446 | 17.09 | 238.00 | 0.001 | 0.600 | 14.51 |
| Adsorption | Elovich Equation | Power Equation | ||||||
| α (µg/g min) | β (g/µg) | R² | RMSE | a | b | R² | RMSE | |
| As(III) | 808,122.16 | 0.129 | 0.830 | 5.166 | 93.36 | 0.062 | 0.842 | 4.979 |
| As(V) | 2,288,305.88 | 0.075 | 0.807 | 10.07 | 166.34 | 0.061 | 0.822 | 9.681 |
| Adsorption | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax (µg/g) | KL (L/µg) | R² | RMSE | KF ((µg/g)(L/µg)1/n) | n | R² | RMSE | |
| As(III) | 694.3 | 1.527 | 0.897 | 3.865 | 502.8 | 1.346 | 0.894 | 3.903 |
| As(V) | 1720.3 | 0.641 | 0.901 | 9.220 | 655.7 | 1.338 | 0.899 | 9.309 |
| Kinetic Models | Non-Linear Equations | References |
|---|---|---|
| Pseudo First-Order | [74,75] | |
| Pseudo Second-Order | [74,75] | |
| Elovich Equation | [76] | |
| Power Equation | [76] | |
| Isotherm Models | Non-Linear Equations | References |
| Langmuir | [74] | |
| Freundlich | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkure, S.; Gamero-Melo, P.; Garrido-Hoyos, S.E.; Reyes-Rosas, A.; Pacheco-Catalán, D.-E.; López-Martínez, A.M. The Development of Fe3O4-Monolithic Resorcinol-Formaldehyde Carbon Xerogels Using Ultrasonic-Assisted Synthesis for Arsenic Removal of Drinking Water. Gels 2023, 9, 618. https://doi.org/10.3390/gels9080618
Khamkure S, Gamero-Melo P, Garrido-Hoyos SE, Reyes-Rosas A, Pacheco-Catalán D-E, López-Martínez AM. The Development of Fe3O4-Monolithic Resorcinol-Formaldehyde Carbon Xerogels Using Ultrasonic-Assisted Synthesis for Arsenic Removal of Drinking Water. Gels. 2023; 9(8):618. https://doi.org/10.3390/gels9080618
Chicago/Turabian StyleKhamkure, Sasirot, Prócoro Gamero-Melo, Sofía Esperanza Garrido-Hoyos, Audberto Reyes-Rosas, Daniella-Esperanza Pacheco-Catalán, and Arely Monserrat López-Martínez. 2023. "The Development of Fe3O4-Monolithic Resorcinol-Formaldehyde Carbon Xerogels Using Ultrasonic-Assisted Synthesis for Arsenic Removal of Drinking Water" Gels 9, no. 8: 618. https://doi.org/10.3390/gels9080618






