Synthesis and Evaluation of Functionalized Polyurethanes for pH-Responsive Delivery of Compounds in Chronic Wounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Synthesized Polymers
2.2. Physicochemical Characterization of Hydrogel Films
2.3. In Vitro Release Study
2.3.1. The Release of Model Compounds
2.3.2. Step pH Release
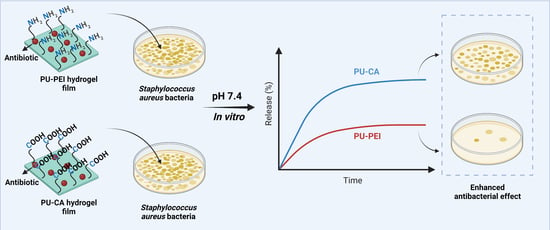
2.3.3. The Release of Antibiotics
2.4. In Vitro Antibacterial Activity of the Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PU−PEI hydrogel
4.3. Synthesis of PU−CA Hydrogel
4.4. Preparation of Hydrogel Films
4.5. Physicochemical Characterization of Polymers
4.6. Physicochemical Characterization of Hydrogel Films
4.7. In Vitro Release Studies
4.8. In Vitro Antibacterial Activity Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. In Seminars in Vascular Surgery; WB Saunders: Philadelphia, PA, USA, 2018; pp. 43–48. [Google Scholar]
- Ferrer-Tasies, L.; Santana, H.; Cabrera-Puig, I.; González-Mira, E.; Ballell-Hosa, L.; Castellar-Álvarez, C.; Córdoba, A.; Merlo-Mas, J.; Gerónimo, H.; Chinea, G. Recombinant human epidermal growth factor/quatsome nanoconjugates: A robust topical delivery system for complex wound healing. Adv. Ther. 2021, 4, 2000260. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [PubMed] [Green Version]
- Boulton, A.J.; Armstrong, D.G.; Hardman, M.J.; Malone, M.; Embil, J.M.; Attinger, C.E.; Lipsky, B.A.; Aragón-Sánchez, J.; Li, H.K.; Schultz, G. Diagnosis and management of diabetic foot infections. Compendia 2020, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Sun, L.; Zhao, Y. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef]
- Shen, S.; Fan, D.; Yuan, Y.; Ma, X.; Zhao, J.; Yang, J. An ultrasmall infinite coordination polymer nanomedicine-composited biomimetic hydrogel for programmed dressing-chemo-low level laser combination therapy of burn wounds. Chem. Eng. J. 2021, 426, 130610. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Piao, Y.; You, H.; Xu, T.; Bei, H.-P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical applications of gelatin methacryloyl hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A textile dressing for temporal and dosage controlled drug delivery. Adv. Funct. Mater. 2017, 27, 1702399. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple stimuli-responsive MXene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ formation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Bayan, M.F.; Marji, S.M.; Salem, M.S.; Begum, M.Y.; Chidambaram, K.; Chandrasekaran, B. Development of Polymeric-Based Formulation as Potential Smart Colonic Drug Delivery System. Polymers 2022, 14, 3697. [Google Scholar] [CrossRef]
- Xue, K.; Wang, X.; Yong, P.W.; Young, D.J.; Wu, Y.L.; Li, Z.; Loh, X.J. Hydrogels as emerging materials for translational biomedicine. Adv. Ther. 2019, 2, 1800088. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Mahajan, A.; Patel, K.; Syed, S.; Acevedo-Jake, A.M.; Kumar, V.A. Materials and cytokines in the healing of diabetic foot ulcers. Adv. Ther. 2021, 4, 2100075. [Google Scholar] [CrossRef]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-regulated drug delivery dermal patch for targeting infected regions in chronic wounds. Lab Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and processable hydrogels based on lignin and hydrophilic polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef]
- Crago, M.; Lee, A.; Farajikhah, S.; Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Winlaw, D.S.; Naficy, S. The evolution of polyurethane heart valve replacements: How chemistry translates to the clinic. Mater. Today Commun. 2022, 33, 104916. [Google Scholar] [CrossRef]
- Naficy, S.; Dehghani, F.; Chew, Y.V.; Hawthorne, W.J.; Le, T.Y.L. Engineering a porous hydrogel-based device for cell transplantation. ACS Appl. Bio Mater. 2020, 3, 1986–1994. [Google Scholar] [CrossRef]
- Javadi, M.; Gu, Q.; Naficy, S.; Farajikhah, S.; Crook, J.M.; Wallace, G.G.; Beirne, S.; Moulton, S.E. Conductive tough hydrogel for bioapplications. Macromol. Biosci. 2018, 18, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, J.E.; Duan, H.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. A highly flexible, physically stable, and selective hydrogel-based hydrogen peroxide sensor. Sens. Actuators B Chem. 2022, 371, 132483. [Google Scholar] [CrossRef]
- Naficy, S.; Oveissi, F.; Patrick, B.; Schindeler, A.; Dehghani, F. Printed, flexible pH sensor hydrogels for wet environments. Adv. Mater. Technol. 2018, 3, 1800137. [Google Scholar] [CrossRef]
- Naficy, S.; Gately, R.; Gorkin III, R.; Xin, H.; Spinks, G.M. 4D printing of reversible shape morphing hydrogel structures. Macromol. Mater. Eng. 2017, 302, 1600212. [Google Scholar] [CrossRef]
- Naficy, S.; Spinks, G.M.; Wallace, G.G. Thin, tough, pH-sensitive hydrogel films with rapid load recovery. ACS Appl. Mater. Interfaces 2014, 6, 4109–4114. [Google Scholar] [CrossRef]
- Naficy, S.; Le, T.Y.L.; Oveissi, F.; Lee, A.; Hung, J.C.; Wise, S.G.; Winlaw, D.S.; Dehghani, F. Highly porous, biocompatible tough hydrogels, processable via gel fiber spinning and 3D gel printing. Adv. Mater. Interfaces 2020, 7, 1901770. [Google Scholar] [CrossRef] [Green Version]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough hydrophilic polyurethane-based hydrogels with mechanical properties similar to human soft tissues. J. Mater. Chem. B 2019, 7, 3512–3519. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Wang, B.; Peng, P.; Gao, C. Physiologically Responsive Polyurethanes for Tissue Repair and Regeneration. Adv. NanoBiomed Res. 2022, 2, 2200061. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Abrami, M.; Grassi, M.; Zoso, A.; Chiono, V.; Ciardelli, G. Dual stimuli-responsive polyurethane-based hydrogels as smart drug delivery carriers for the advanced treatment of chronic skin wounds. Bioact. Mater. 2021, 6, 3013–3024. [Google Scholar] [CrossRef]
- Hung, W.-C.; Cherng, J.-Y. Maleimide-Functionalized PEI600 Grafted Polyurethane: Synthesis, Nano-Complex Formation with DNA and Thiol-Conjugation of the Complexes for Dual DNA Transfection. Polymers 2015, 7, 2131–2145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Jiang, H.; Li, Y.; Pang, J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021, 362, 130242. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]











| Ciprofloxacin | EtOH Water (Control) | |||||
|---|---|---|---|---|---|---|
| Bacteria | MIC (µg/mL) | MBC (µg/mL) | MIC (%) | MBC (%) | ||
| E. coli | 0.0313 | 0.0313 | ≤4 | 0.063 | 12.5 | ≤4 |
| S. aureus | 1 | 1 | ≤4 | 12.5 | 12.5 | ≤4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Crago, M.; Schofield, T.; Zeng, H.; Vyas, H.K.N.; Müllner, M.; Mai-Prochnow, A.; Farajikhah, S.; Naficy, S.; Dehghani, F.; et al. Synthesis and Evaluation of Functionalized Polyurethanes for pH-Responsive Delivery of Compounds in Chronic Wounds. Gels 2023, 9, 611. https://doi.org/10.3390/gels9080611
Li Z, Crago M, Schofield T, Zeng H, Vyas HKN, Müllner M, Mai-Prochnow A, Farajikhah S, Naficy S, Dehghani F, et al. Synthesis and Evaluation of Functionalized Polyurethanes for pH-Responsive Delivery of Compounds in Chronic Wounds. Gels. 2023; 9(8):611. https://doi.org/10.3390/gels9080611
Chicago/Turabian StyleLi, Zhongyan, Matthew Crago, Timothy Schofield, Haoxiang Zeng, Heema Kumari Nilesh Vyas, Markus Müllner, Anne Mai-Prochnow, Syamak Farajikhah, Sina Naficy, Fariba Dehghani, and et al. 2023. "Synthesis and Evaluation of Functionalized Polyurethanes for pH-Responsive Delivery of Compounds in Chronic Wounds" Gels 9, no. 8: 611. https://doi.org/10.3390/gels9080611