Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Ciprofloxacin Nanoemulsion (CIP.HCl NE)
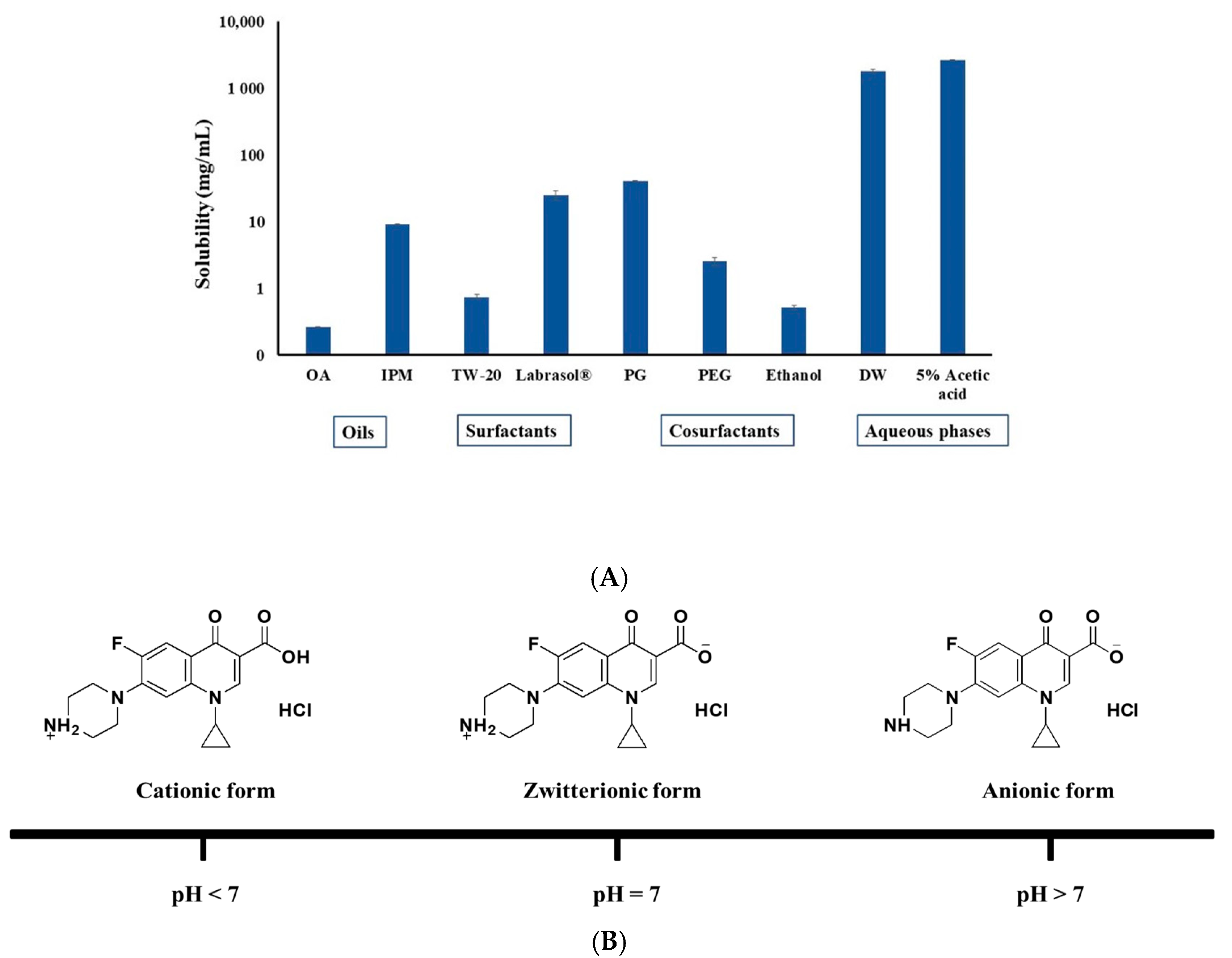
2.1.1. Selection of CIP.HCl NE Components
2.1.2. Characterization of CIP.HCl NE
Droplet Size, PDI, and ζ-Potential
Morphology of CIP.HCl NE
2.1.3. CIP.HCl Content
2.1.4. Stability Studies of CIP.HCl NE
Thermodynamic Stability
Chemical Stability
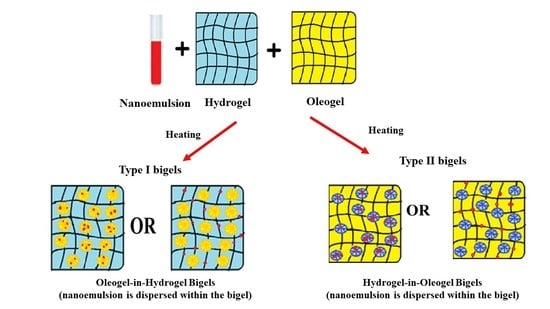
2.2. Preparation and Physical Appearance of Bigels
2.3. Characterization of Bigels
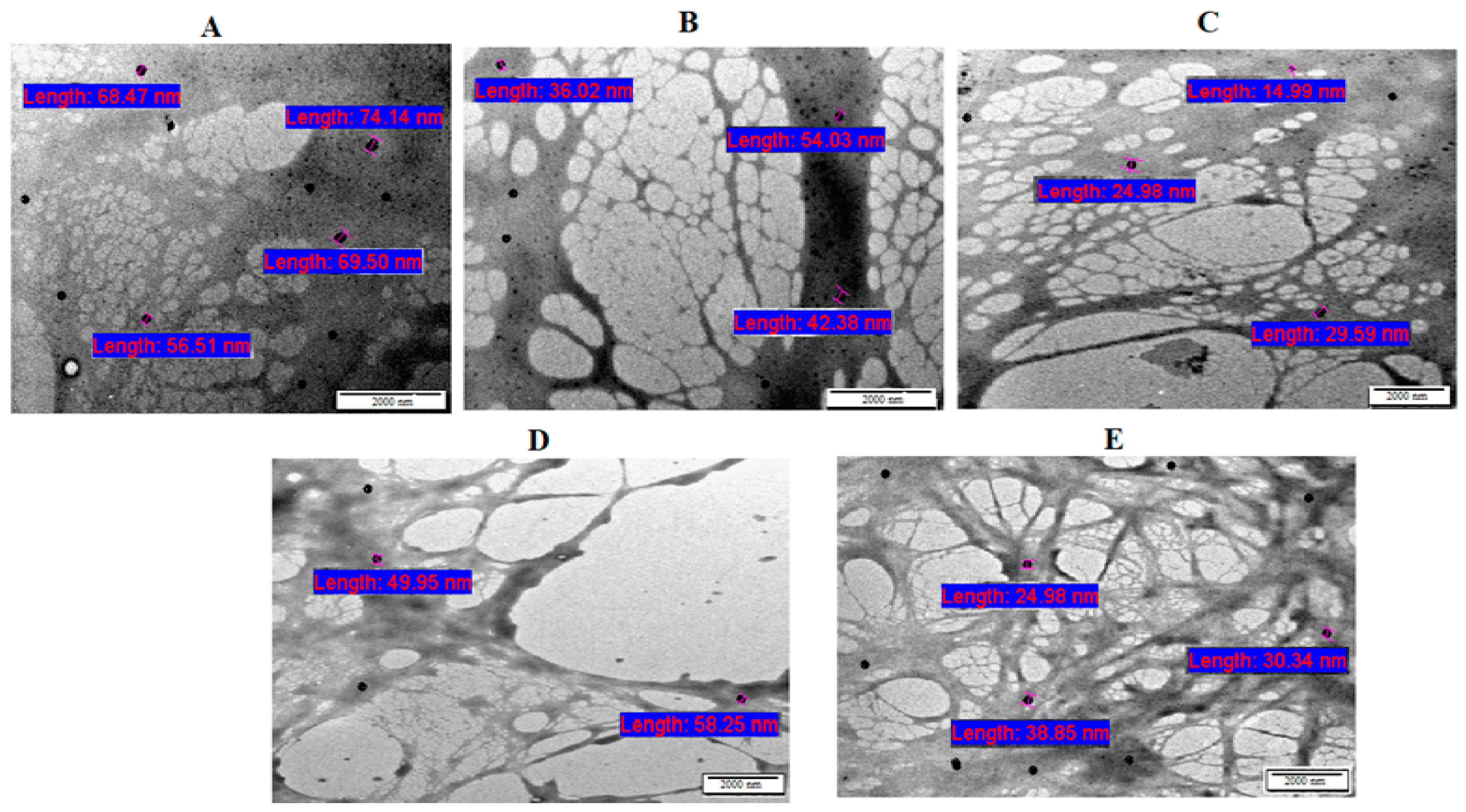
2.3.1. Microstructural Characterization
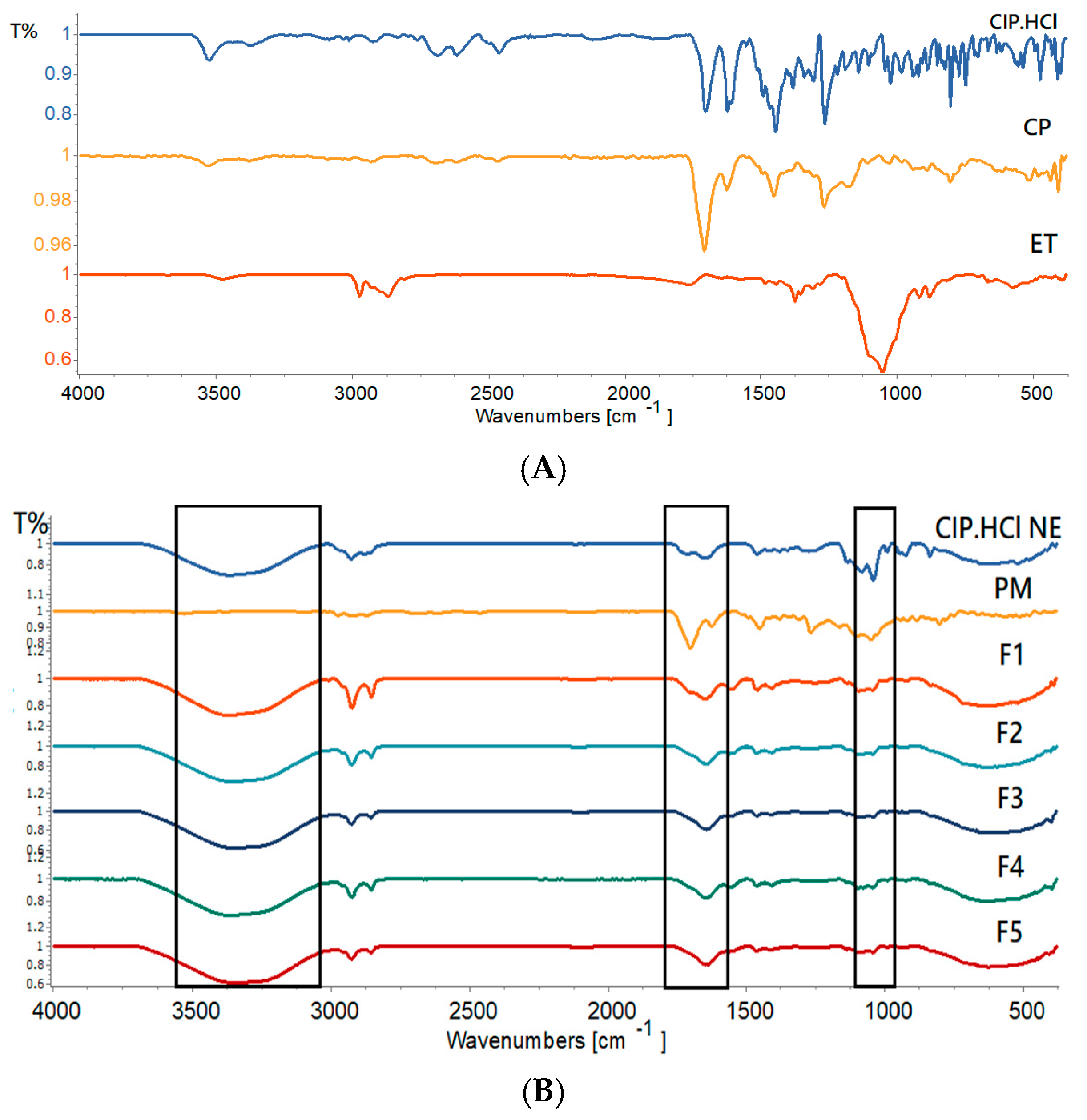
2.3.2. Fourier Transform Infrared (FTIR)
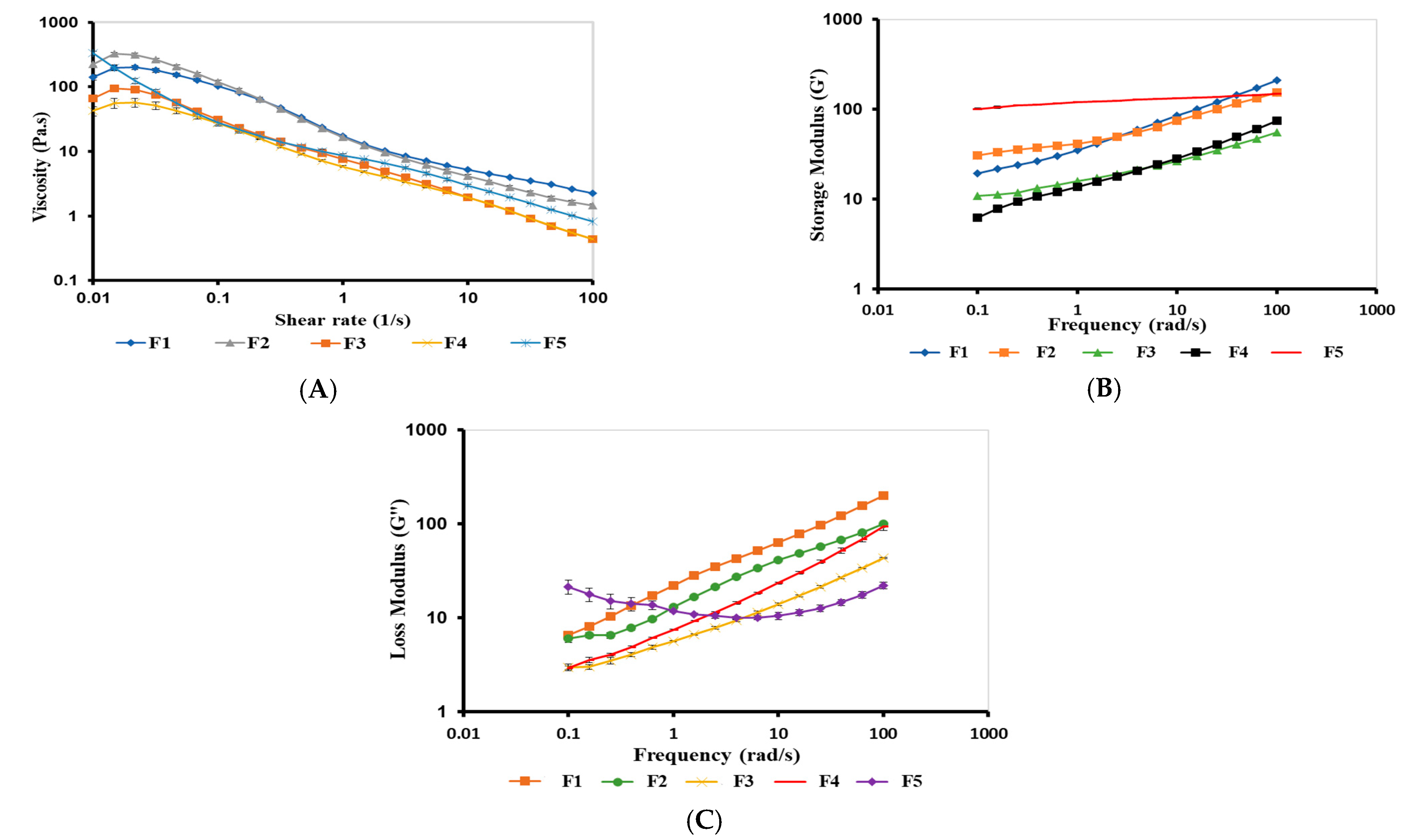
2.3.3. Rheological Studies
Viscosity of Bigels
Strain-Sweep of Bigels
Frequency Sweep of Bigels
2.3.4. In Vitro Release Studies
2.3.5. Mechanism of CIP.HCl Release
2.3.6. Antimicrobial Study
Well Diffusion Methods
Ex Vivo Skin Infection Model
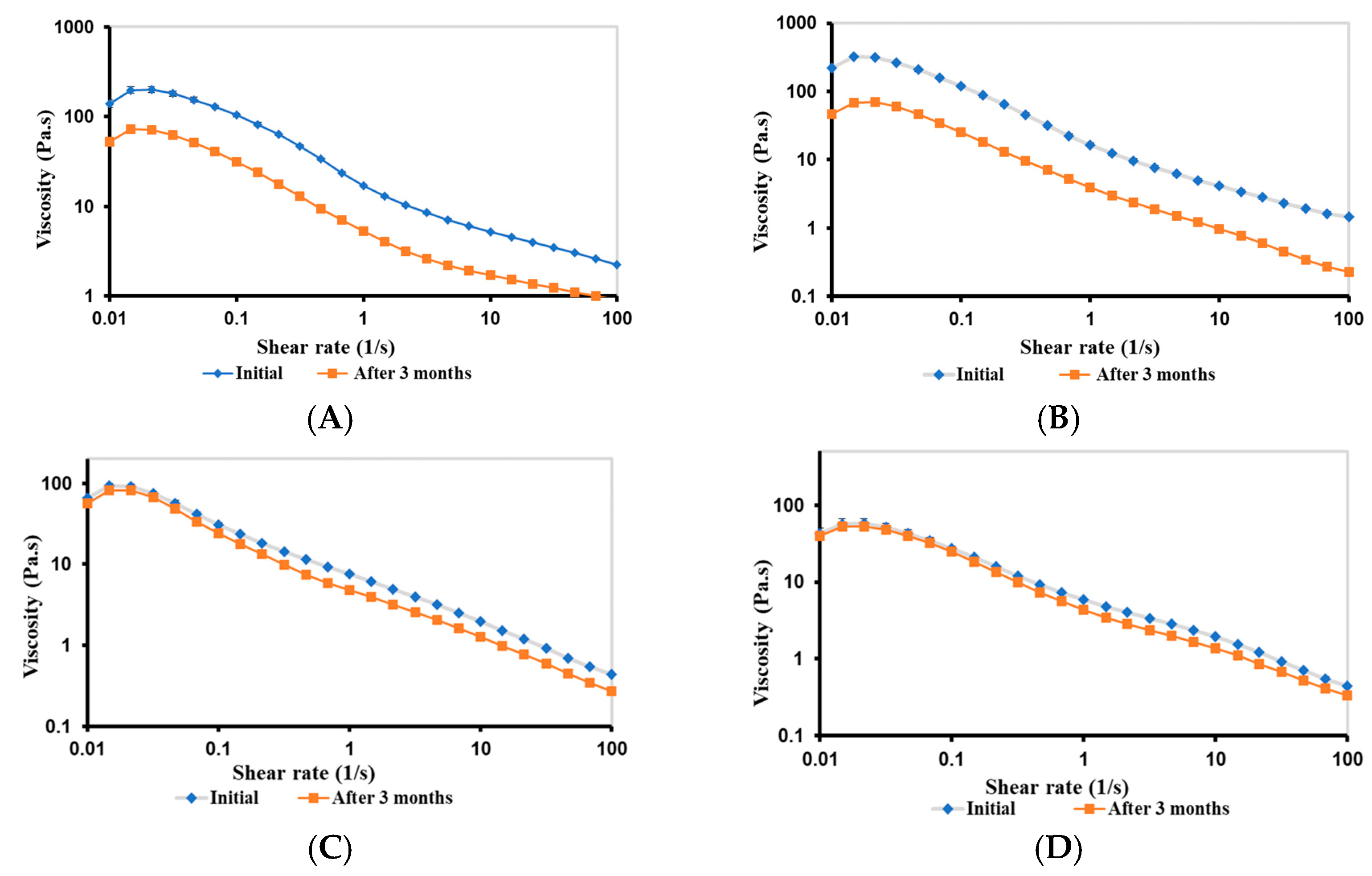
2.3.7. Rheological Stability of Bigels
Viscosity of Bigels
Viscoelastic Properties of Bigels
2.3.8. Physicochemical Stability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Ciprofloxacin HCl Nanoemulsion (CIP.HCl NE)
Solubility of CIP.HCl
Preparation of CIP.HCl NE
4.2.2. Characterization of CIP.HCl NE
Particle size, Polydispersity Index, and Zeta Potential
Morphology of CIP.HCl NE
4.2.3. High Performance Liquid Chromatography
4.2.4. Determination of CIP.HCl in Nanoemulsion
4.2.5. Stability Studies of CIP.HCl NE
Thermodynamic Stability Studies
Chemical Stability
4.2.6. Preparation of Bigels
Preparation of Bare WH
Preparation of Bare OL
Preparation of Bigels
4.2.7. Characterization of CIP.HCl Bigels
Physical Appearance
Microstructural Characterization
Fourier Transform Infrared (FTIR)
4.2.8. Determination of CIP.HCl in Bigels
4.2.9. Rheological Studies
Viscosity Curves
Strain-Sweep
Frequency-Sweep
4.2.10. In Vitro Release
4.2.11. Kinetic Release Analysis
4.2.12. Antimicrobial Studies
Microorganisms
Well-Diffusion Method
Ex Vivo Skin Infection Model
4.2.13. Stability Studies of Bigels
Rheological Stability
Physicochemical Stability
4.2.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahraman, E.; Kaykın, M.; Şahin Bektay, H.; Güngör, S. Recent advances on topical application of ceramides to restore barrier function of skin. Cosmetics 2019, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Tambunlertchai, S.; Geary, S.M.; Salem, A.K. Skin penetration enhancement strategies used in the development of melanoma topical treatments. AAPS J. 2021, 23, 19. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Al-Assaf, S.F.; Hamed, R. Recent exploration of nanoemulsions for drugs and cosmeceuticals delivery. J. Cosmet. Dermatol. 2021, 21, 3729–3740. [Google Scholar] [CrossRef]
- Hamed, R.; Basil, M.; AlBaraghthi, T.; Sunoqrot, S.; Tarawneh, O. Nanoemulsion-based gel formulation of diclofenac diethylamine: Design, optimization, rheological behavior and in vitro diffusion studies. Pharm. Dev. Technol. 2016, 21, 980–989. [Google Scholar] [CrossRef]
- Hamed, R.; Farhan, A.; Abu-Huwaij, R.; Mahmoud, N.N.; Kamal, A. Lidocaine microemulsion-laden organogels as lipid-based systems for topical delivery. J. Pharm. Innov. 2019, 15, 521–534. [Google Scholar] [CrossRef]
- Hamed, R.; Mahmoud, N.N.; Alnadi, S.H.; Alkilani, A.Z.; Hussein, G. Diclofenac diethylamine nanosystems-loaded bigels for topical delivery: Development, rheological characterization, and release studies. Drug Dev. Ind. Pharm. 2020, 46, 1705–1715. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as drug delivery systems: From their components to their applications. Drug Discov. Today 2021, 27, 1008–1026. [Google Scholar] [CrossRef]
- Mazurkeviciute, A.; Ramanauskiene, K.; Ivaskiene, M.; Grigonis, A.; Briedis, V. Topical antifungal bigels: Formulation, characterization and evaluation. Acta Pharm. 2018, 68, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of bigels based on stearic acid–rice bran oil oleogels and tamarind gum hydrogels for controlled delivery applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Alhusban, A.A.; Tarawneh, O.A.; Dawabsheh, S.O.; Alhusban, A.A.; Abumhareb, F.W. Liquid chromatography–tandem mass spectrometry for rapid and selective simultaneous determination of fluoroquinolones level in human aqueous humor. J. Pharmacol. Toxicol. Methods 2019, 97, 36–43. [Google Scholar] [CrossRef]
- Nugrahani, I.; Tjengal, B.; Gusdinar, T.; Horikawa, A.; Uekusa, H. A comprehensive study of a new 1.75 hydrate of ciprofloxacin salicylate: SCXRD structure determination, solid characterization, water stability, solubility, and dissolution study. Crystals 2020, 10, 349. [Google Scholar] [CrossRef]
- Osonwa, U.E.; Ugochukwu, J.I.; Ajaegbu, E.E.; Chukwu, K.I.; Azevedo, R.B.; Esimone, C.O. Enhancement of antibacterial activity of ciprofloxacin hydrochloride by complexation with sodium cholate. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 233–237. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Hamed, R.; Al-Marabeh, S.; Kamal, A.; Abu-Huwaij, R.; Hamad, I. Nanoemulsion-based film formulation for transdermal delivery of carvedilol. J Drug Deliv. Sci. Technoly 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Caço, A.; Varanda, F.; Pratas, M.; Dias, A.; Dohrn, R.; Marrucho, I. Solubility of Antibiotics in Different Solvents. Part II. Non-Hydrochloride Forms of Tetracycline and Ciprofloxacin. Ind. Eng. Chem. 2008, 47, 8083–8089. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Varanda, F.; Pratas de Melo, M.J.; Caco, A.I.; Dohrn, R.; Makrydaki, F.A.; Voutsas, E.; Tassios, D.; Marrucho, I.M. Solubility of antibiotics in different solvents. 1. Hydrochloride forms of tetracycline, moxifloxacin, and ciprofloxacin. Ind. Eng. Chem. Res. 2006, 45, 6368–6374. [Google Scholar] [CrossRef]
- Jusril, N.A.; Abu Bakar, S.I.; Khalil, K.A.; Md Saad, W.M.; Wen, N.K.; Adenan, M.I. Development and Optimization of Nanoemulsion from Ethanolic Extract of Centella asiatica (NanoSECA) Using D-Optimal Mixture Design to Improve Blood-Brain Barrier Permeability. Evid. Based Complement. Altern. Med. 2022, 2022, 3483511. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Sila-on, W.; Vardhanabhuti, N.; Ongpipattanakul, B.; Kulvanich, P. Influence of incorporation methods on partitioning behavior of lipophilic drugs into various phases of a parenteral lipid emulsion. AAPS PharmSciTech 2008, 9, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of ethyl cellulose-based formulations: A perspective on the novel technical methods. Food Rev. Int. 2022, 38, 685–732. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. Ethylcellulose oleogels: Structure, functionality, and food applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 1–56. [Google Scholar]
- Lai, H.L.; Pitt, K.; Craig, D.Q. Characterisation of the thermal properties of ethylcellulose using differential scanning and quasi-isothermal calorimetric approaches. Int. J. Pharm. 2010, 386, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation between rheological properties and in vitro drug release from penetration enhancer-loaded Carbopol® gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Zetzl, A.K.; Gravelle, A.J.; Kurylowicz, M.; Dutcher, J.; Barbut, S.; Marangoni, A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Struct. 2014, 2, 27–40. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Prasanth, V.; Chakraborty, A.; Mathew, S.T.; Mathappan, R.; Kamalakkannan, V. Formulation and evaluation of Salbutamol sulphate microspheres by solvent evaporation method. J. Appl. Pharm. Sci. 2011, 2011, 133–137. [Google Scholar]
- Chakraborty, S.; Khandai, M.; Sharma, A.; Khanam, N.; Patra, C.; Dinda, S.; Sen, K. Preparation, and evaluation of algino-pectinate bioadhesive microspheres: An investigation of the effects of polymers using multiple comparison analysis. Acta Pharm. 2010, 60, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Pani, N.R.; Sahoo, S.K. Microemulsion based topical hydrogel of sertaconazole: Formulation, characterization and evaluation. Colloids Surf. B. Biointerfaces 2014, 120, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Zulfakar, M.H. Novel fish oil-based bigel system for controlled drug delivery and its influence on immunomodulatory activity of imiquimod against skin cancer. Pharm. Res. 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.; Mishra, S.; Naik, S.; Nanda, U. FTIR and Raman spectroscopy as a tool for analyzing sustained release hydrogel of ciprofloxacin/Carbopol polymer. Int. J. Pharm. Sci. Res. 2011, 2, 268–277. [Google Scholar]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.; Jana, S. Characterization of physicochemical and thermal properties of biofield treated ethyl cellulose and methyl cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. [Google Scholar]
- Musto, P.; Karasz, F.; MacKnight, W. Hydrogen bonding in polybenzimidazole/polyimide systems: A Fourier-transform infra-red investigation using low-molecular-weight monofunctional probes. Polymer 1989, 30, 1012–1021. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Hamed, R.; AbuRezeq, A.a.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf. B. Biointerfaces 2014, 123, 582–592. [Google Scholar] [CrossRef]
- Ilomuanya, M.; Ajayi, T.; Cardoso-Daodu, I.; Akhimien, T.; Adeyinka, O.; Aghaizu, C. Formulation and Evaluation of Polyherbal Antioxidant Face Cream Containing Ethanol Extracts of Psidium Guajava and Ocimum Gratissimum. Niger. J. Pharm. Sci. 2018, 14, 61–68. [Google Scholar]
- Ortan, A.; Parvu, C.D.; Ghica, M.V.; Popescu, L.M.; Ionita, L. Rheological study of a liposomal hydrogel based on carbopol. Rom. Biotechnol. Lett. 2011, 16, 47–54. [Google Scholar]
- Hamed, R.; Al-Adhami, Y.; Abu-Huwaij, R. Concentration of a microemulsion influences the mechanical properties of ibuprofen in situ microgels. Int. J. Pharm. 2019, 570, 118684. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Behera, B.; Singh, V.; Kulanthaivel, S.; Bhattacharya, M.; Paramanik, K.; Banerjee, I.; Pal, K. Physical and mechanical properties of sunflower oil and synthetic polymers based bigels for the delivery of nitroimidazole antibiotic–A therapeutic approach for controlled drug delivery. Eur. Polym. J. 2015, 64, 253–264. [Google Scholar] [CrossRef]
- Rothan, A.; Muller, R.; Hebraud, P.; Castro, M.; Bouquey, M.; Serra, C. Unusual time dependent rheological behavior of a concentrated suspension. Appl. Rheol. 2017, 27, 20–26. [Google Scholar]
- Hamed, R.; Abu Kwiak, A.D.; Al-Adhami, Y.; Hammad, A.M.; Obaidat, R.; Abusara, O.H.; Huwaij, R.A. Microemulsions as Lipid Nanosystems Loaded into Thermoresponsive In Situ Microgels for Local Ocular Delivery of Prednisolone. Pharmaceutics 2022, 14, 1975. [Google Scholar] [CrossRef]
- Mallah, E. Relative bioavailability of ciprofloxacin doses (750 and 1000) mg in healthy male volunteers by using HPLC method. J. Bioequiv. Stud. 2015, 1, 101. [Google Scholar]
- Mahmoud, N.N.; Hamed, R.; Khalil, E.A. Colloidal stability and rheological properties of gold nanoparticle–loaded polymeric hydrogels: Impact of nanoparticle’s shape, surface modification, and concentration. Colloid. Polym. Sci. 2020, 298, 989–999. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Bauer, A. Antibiotic susceptibility testing by a standardized single diffusion method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Rubinchik, E.; Pasetka, C. Ex vivo skin infection model. Antimicrob. Pept. Methods Protoc. 2010, 618, 359–369. [Google Scholar]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef] [PubMed]






| Time | Initial | 7 Days | 14 Days | 21 Days | 28 Days |
|---|---|---|---|---|---|
| Droplet size (nm) | 17.27 ± 2.74 | 11.19 ± 0.11 | 22.88 ± 1.91 | 11.19 ± 0.01 | 17.73 ± 0.06 |
| PDI | 0.21 ± 0.01 | 0.40 ± 0.02 | 0.40 ± 0.01 | 0.48 ± 0.03 | 0.49 ± 0.01 |
| ζ-potential (mV) | 0.70 ± 0.01 | 0.80 ± 0.10 | 0.50 ± 0.01 | 0.61 ± 0.02 | 1.57 ± 0.23 |
| Time | 2–8 °C | Room Temperature (22 °C) | 30 °C/65% RH | 40 °C/75% RH | |
|---|---|---|---|---|---|
| Storage Condition | |||||
| Initial (%) | 100.6 ± 2.9 | 100.6 ± 2.9 | 100.6 ± 2.9 | 100.6 ± 2.9 | |
| 1 month (%) | 98.9 ± 1.6 | 100.8 ± 1.0 | 101.5 ± 1.3 | 100.3 ± 1.3 | |
| 2 months (%) | 96.5 ± 4.2 | 103.8 ± 2.7 | 101.2 ± 0.4 | 104.1 ± 3.6 | |
| 3 months (%) | 99.4 ± 1.7 | 102.1 ± 0.7 | 102.3 ± 0.3 | 105.6 ± 1.2 | |
| Bigels | O-H Stretching Vibration Peak (cm−1) | C=O Stretching Vibration Peak (cm−1) | C-O-C Stretching Peak (cm−1) | CH Stretching Vibration Peak (cm−1) |
|---|---|---|---|---|
| F1 | 3367 | 1652 | 1043 | 2853–2923 |
| F2 | 3351 | 1645 | 1043 | 2853–2923 |
| F3 | 3348 | 1642 | 1043 | 2854–2924 |
| F4 | 3360 | 1642 | 1043 | 2854–2924 |
| F5 | 3344 | 1636 | 1042 | 2854–2924 |
| Bigels | LVR (%) | Applied Strain within the LVR (%) | Critical Strain (γC, %) |
|---|---|---|---|
| F1 | 0.016–5.500 | 0.10 | 5.500 |
| F2 | 0.010–9.700 | 0.10 | 9.700 |
| F3 | 0.010–10.000 | 0.10 | 10.000 |
| F4 | 0.010–10.000 | 0.10 | 10.000 |
| F5 | 0.010–1.000 | 0.10 | 1.000 |
| Bigels | K (h−1 ) | n | R2 |
|---|---|---|---|
| F1 | 16.73 | 1.07 | 0.9988 |
| F2 | 19.96 | 1.01 | 0.9973 |
| F3 | 18.05 | 1.09 | 0.9988 |
| F4 | 19.02 | 1.03 | 0.9976 |
| F5 | 23.47 | 1.12 | 0.9895 |
| Sample | Tested Sample | Gram-Negative Bacteria | Gram-Positive Bacteria | |||
|---|---|---|---|---|---|---|
| B. subtilis (mm) | S. aureus (mm) | E. coli (mm) | P. mirabilis (mm) | P. aeruginosa (mm) | ||
| 1 | 0.3% Ciprocin eye/ear drops | 43.5 ± 2.1 *** | 37.5 ± 2.1 ** | 36.5 ± 2.1 | 38.5 ± 2.1 ** | 38.0 ± 0.0 ** |
| 2 | 1.5% CIP.HCl solution | 49.5 ± 2.1 *** | 42.0 ± 0.7 *** | 40.5 ± 0.7 | 42.5 ± 0.7 *** | 40.0 ± 0.0 *** |
| 3 | 1.5% CIP.HCl NE | 48.0 ± 0.0 *** | 40.0 ± 0.0 ** | 36.0 ± 1.4 | 36.5 ± 0.7 ** | 41.0 ± 1.4 *** |
| 3N | Blank NE | 24.5 ± 2.1 | 14.0 ± 0.0 | 19.5 ± 0.7 | 23.0 ± 0.0 | 12.0 ± 0.0 |
| 4 | F1 | 36.0 ± 0.0 ** | 28.5 ± 0.7 ** | 30.0 ± 2.8 ** | 29.0 ± 0.7 ** | 32.0 ± 0.0 ** |
| 4N | Blank of F1 | 8.0 ± 0.0 | 9.0 ± 0.0 | NZ | NZ | NZ |
| 5 | F2 | 39.5 ± 0.7 ** | 29.5 ± 0.7 ** | 32.5 ± 0.7 ** | 34.0 ± 1.4 ** | 35.5 ± 2.1 ** |
| 5N | Blank of F2 | 7.0 ± 0.0 | NZ | NZ | NZ | NZ |
| 6 | F3 | 40.0 ± 0.0 ** | 31.0 ± 0.0 | 32.0 ± 0.0 | 33.0 ± 0.7 | 34.0 ± 1.4 |
| 6N | Blank of F3 | 8.0 ± 0.0 | NZ | NZ | NZ | NZ |
| 7 | F4 | 38.5 ± 0.0 ** | 29.0 ± 0.0 | 31.5 ± 2.8 * | 31.0 ± 0.7 * | 34.5 ± 0.7 ** |
| 7N | Blank of F4 | 9.0 ± 0.0 | NZ | 6.0 ± 0.0 | NZ | NZ |
| 8 | F5 | 42.0 ± 0.7 ** | 33.5 ± 0.7 ** | 35.0 ± 0.7 ** | 36.0 ± 0.7 ** | 36.5 ± 2.8 ** |
| 8N | Blank of F5 | 9.0 ± 0.0 | 7.0 ± 0.0 | NZ | NZ | NZ |
| Treatment | Gram-Negative Bacteria | Gram-Positive Bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | P. mirabilis | B. subtilis | S. aureus | ||||||
| Mean log10 CFU/Skin ± SD after 24 h Post-Treatment | Log10 CFU/Skin Reduction vs. Control | Mean log10 CFU/Skin ± SD after 24 h Post-Treatment | Log10 CFU/Skin Reduction vs. Control | Mean log10 CFU/Skin ± SD after 24 h Post-Treatment | Log10 CFU/Skin Reduction vs. Control | Mean log10 CFU/Skin ± SD after 24 h Post-Treatment | Log10 CFU/Skin Reduction vs. Control | Mean log10 CFU/skin ± SD after 24 h Post-Treatment | Log10 CFU/Skin Reduction vs. Control | |
| No treatment (positive control) | 9.74 ± 1.75 | N/A | 9.63 ± 1.64 | N/A | 9.55 ± 1.76 | N/A | 9.76 ± 1.78 | N/A | 9.58 ± 1.96 | N/A |
| Treatment 1 (F3) | 2.70 ± 0.02 | −7.04 * | 2.30 ± 0.02 | −7.33 * | NC | −9.55 * | 4.41 ± 0.02 | −5.35 * | NC | −9.58 * |
| Treatment 2 (F5) | NC | −9.74 * | NC | −9.63 * | NC | −9.55 * | NC | −9.76 *,# | NC | −9.58 * |
| Negative control | NC | N/A | NC | N/A | NC | N/A | NC | N/A | NC | N/A |
| Bigels | Initial | One Month | Two Months | Three Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | ||
| F2 | 6.5 ± 0.1 | 6.4 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.1 | 6.0 ± 0.3 | 6.1 ± 0.2 | 6.1 ± 0.1 | 6.1 ± 0.1 | 6.1 ± 0.3 | 6.2 ± 0.2 | 6.1 ± 0.0 | 6.1 ± 0.0 | 6.2 ± 0.2 |
| F3 | 6.5 ± 0.2 | 6.0 ± 0.1 | 5.9 ± 0.0 | 5.9 ± 0.2 | 5.9 ± 0.0 | 5.9 ± 0.1 | 5.9 ± 0.0 | 6.0 ± 0.2 | 5.8 ± 0.0 | 5.9 ± 0.1 | 5.9 ± 0.0 | 5.9 ± 0.1 | 5.9 ± 0.0 |
| F4 | 6.5 ± 0.0 | 5.9 ± 0.0 | 6.1 ± 0.0 | 6.0 ± 0.0 | 6.2 ± 0.0 | 6.3 ± 0.0 | 6.2 ± 0.2 | 6.1 ± 0.0 | 6.1 ± 0.1 | 6.1 ± 0.0 | 6.3 ± 0.2 | 6.1 ± 0.2 | 6.1 ± 0.1 |
| F5 | 6.5 ± 0.0 | 5.9 ± 0.0 | 5.9 ± 0.2 | 5.9 ± 0.0 | 5.7 ± 0.1 | 6.1 ± 0.0 | 6.0 ± 0.2 | 6.0 ± 0.2 | 6.0 ± 0.2 | 6.0 ± 0.1 | 6.0 ± 0.1 | 5.9 ± 0.0 | 6.1 ± 0.1 |
| Bigels | Initial | One Month | Two Months | Three Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | 2–8 °C | 22 °C | 30 °C/ 65% RH | 40 °C/ 75% RH | ||
| F2 | 92.1 ± 1.1 | 85.6 ± 4.0 | 86.8 ± 3.3 | 82.2 ± 2.5 | 80.8 ± 4.2 | 85.2 ± 2.6 | 84.3 ± 3.5 | 82.3 ± 2.0 | 79.2 ± 3.7 | 82.8 ± 4.1 | 83.3 ± 3.6 | 80.1 ± 2.6 | 77.2 ± 3.8 |
| F3 | 93.6 ± 2.9 | 91.5 ± 2.1 | 88.6 ± 1.4 | 86.4 ± 0.7 | 85.0 ± 4.9 | 90.5 ± 4.0 | 88.7 ± 2.0 | 86.0 ± 3.2 | 84.6 ± 0.5 | 90.7 ± 0.5 | 86.7 ± 2.9 | 85.7 ± 3.2 | 83.9 ± 5.7 |
| F4 | 93.6 ± 4.3 | 88.6 ± 2.3 | 88.6 ± 3.2 | 84.1 ± 1.2 | 82.5 ± 3.3 | 90.4 ± 3.0 | 87.3 ± 2.6 | 83.4 ± 4.3 | 81.4 ± 1.9 | 87.4 ± 2.7 | 85.1 ± 4.8 | 80.6 ± 3.7 | 80.3 ± 2.1 |
| F5 | 94.1 ± 2.6 | 91.2 ± 2.4 | 91.1 ± 2.3 | 92.3 ± 1.4 | 90.6 ± 3.1 | 90.9 ± 0.2 | 91.9 ± 5.0 | 91.4 ± 5.2 | 91.0 ± 3.2 | 90.4 ± 3.3 | 92.2 ± 3.6 | 92.4 ± 3.2 | 89.8 ± 2.3 |
| Type I Bigels | OL:WH Ratio | CIP.HCl NE (mL) | WH Phase (g/mL) | OL Phase (g/mL) | The Phase in Which CIP.HCl NE Was Dispersed | ||
|---|---|---|---|---|---|---|---|
| CP (g) | DW (mL) | EC (g) | OA (mL) | ||||
| F1 | 1:1 | 20 | 0.20 | 40.0 | 4.44 | 40.0 | OL |
| F2 | 1:2 | 20 | 0.27 | 53.3 | 2.97 | 26.7 | OL |
| F3 | 1:4 | 20 | 0.32 | 64.0 | 1.78 | 16.0 | OL |
| F4 | 1:2 | 20 | 0.27 | 53.3 | 2.97 | 26.7 | WH |
| F5 | 1:4 | 20 | 0.32 | 64.0 | 1.78 | 16.0 | WH |
| Type II Bigels | WH:OL Ratio | CIP.HCl NE (mL) | WH Phase (g/mL) | OL Phase (g/mL) | The Phase in Which CIP.HCl NE Was Dispersed | ||
|---|---|---|---|---|---|---|---|
| CP (g) | DW (mL) | EC (g) | OA (mL) | ||||
| F6 | 1:1 | 20 | 0.20 | 40 | 4.44 | 40 | WH |
| F7 | 1:2 | 20 | 0.13 | 26.7 | 5.92 | 53.3 | WH |
| F8 | 1:4 | 20 | 0.08 | 16.0 | 7.11 | 64.0 | WH |
| F9 | 1:2 | 20 | 0.13 | 26.7 | 5.92 | 53.3 | OL |
| F10 | 1:4 | 20 | 0.08 | 16.0 | 7.11 | 64.0 | OL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, R.; Abu Alata, W.; Abu-Sini, M.; Abulebdah, D.H.; Hammad, A.M.; Aburayya, R. Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels. Gels 2023, 9, 592. https://doi.org/10.3390/gels9070592
Hamed R, Abu Alata W, Abu-Sini M, Abulebdah DH, Hammad AM, Aburayya R. Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels. Gels. 2023; 9(7):592. https://doi.org/10.3390/gels9070592
Chicago/Turabian StyleHamed, Rania, Wala’a Abu Alata, Mohammad Abu-Sini, Dina H. Abulebdah, Alaa M. Hammad, and Rafa Aburayya. 2023. "Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels" Gels 9, no. 7: 592. https://doi.org/10.3390/gels9070592