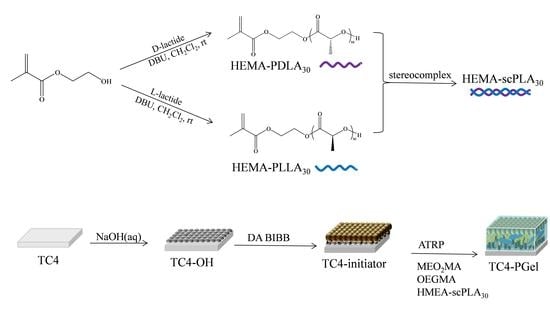
Preparation and Properties of Physical Gel on Medical Titanium Alloy Surface
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure Characterization of Macromolecular Monomers and Initiators
2.3. Micro and Macro Analysis of Initiator Surface Morphology
2.4. X-ray Diffraction Analysis of Physical Gels
2.5. Morphological Analysis of Physical Gels
2.6. Mechanical Properties of Physical Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of TC4-OH
4.2.2. Preparation of TC4-Initiator
4.2.3. Synthesis of Macromolecule HEMA-PLLA29(PDLA29)
4.2.4. Preparation of Hydrogels on TC4
4.3. Structure Characterization
4.4. X-ray Diffraction Analysis of Physical Gels
4.5. The Scanning Electron Microscope (SEM) Analysis of the Initiators and Physical Gels
4.6. Mechanical Properties Analysis of the Physical Gels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanciu, E.-M.; Florido-Suarez, N.; Socorro-Perdomo, P.; Mirza-Rosca, J. Osseo-Integration Improvement of Additive Manufactured Dental Alloys. Microsc. Microanal. 2021, 27, 2388–2391. [Google Scholar] [CrossRef]
- Zapata, J.M.; Leal, E.; Hunter, R.; de Souza, R.F.; Borie, E. Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis. Materials 2022, 15, 8903. [Google Scholar] [CrossRef]
- DeFlorio, W.; Crawford, K.; Liu, S.; Hua, Y.; Cisneros-Zevallos, L.; Akbulut, M. Fluorine-Free, Facile, Fabrication of Bacterial Antifouling Titanium Alloy Ti6Al4V Surfaces for Surgically Implanted Devices. Surf. Coat. Technol. 2022, 443, 128580–128590. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Z.; Zhao, N.; Liu, G.; Zhou, F.; Liu, W. Exploration on Aqueous Lubrication of Polymeric Microgels between Titanium Alloy Contacts. ACS Omega 2021, 6, 32178–32185. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Fukazawa, K.; Huang, N.; Ishihara, K. Effects of 3,4-Dihydrophenyl Groups in Water-Soluble Phospholipid Polymer on Stable Surface Modification of Titanium Alloy. Colloids Surf. B Biointerfaces 2011, 88, 215–220. [Google Scholar] [CrossRef]
- Grygier, D.; Kujawa, M.; Kowalewski, P. Deposition of Biocompatible Polymers by 3D Printing (FDM) on Titanium Alloy. Polymer 2022, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, J.; Peng, H.; Shu, X.; Chen, L.; Guo, H. Tightly Adhered Silk Fibroin Coatings on Ti6Al4V Biometals for Improved Wettability and Compatible Mechanical Properties. Mater. Des. 2019, 175, 107825–107833. [Google Scholar] [CrossRef]
- Rodriguez Lopez, A.L.; Lee, M.R.; Ortiz, B.J.; Gastfriend, B.D.; Whitehead, R.; Lynn, D.M.; Palecek, S.P. Preventing S. aureus Biofilm Formation on Titanium Surfaces by the Release of Antimicrobial β-Peptides from Polyelectrolyte Multilayers. Acta Biomater. 2019, 93, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Ramamurty, U. Boron Modified Titanium Alloys. Progress. Mater. Sci. 2020, 111, 100653–100706. [Google Scholar] [CrossRef]
- Gunay-Bulutsuz, A.; Berrak, O.; Yeprem, H.A.; Arisan, E.D.; Yurci, M.E. Biological Responses of Ultrafine Grained Pure Titanium and Their Sand Blasted Surfaces. Mater. Sci. Eng. C 2018, 91, 382–388. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, J.; Liu, X. Enhanced Antioxidant Capability and Osteogenic Property of Medical Titanium by Cerium Plasma Immersion Ion Implantation. Surf. Interfaces 2021, 26, 101402–101409. [Google Scholar] [CrossRef]
- Singh, J.; Chatha, S.S.; Singh, H. Characterization and Corrosion Behavior of Plasma Sprayed Calcium Silicate Reinforced Hydroxyapatite Composite Coatings for Medical Implant Applications. Ceram. Int. 2021, 47, 782–792. [Google Scholar] [CrossRef]
- Palanivelu, R.; Ruban Kumar, A. Scratch and Wear Behaviour of Plasma Sprayed Nano Ceramics Bilayer Al2O3-13 wt%TiO2/Hydroxyapatite Coated on Medical Grade Titanium Substrates in SBF Environment. Appl. Surf. Sci. 2014, 315, 372–379. [Google Scholar] [CrossRef]
- Hung, K.Y.; Lin, Y.C.; Feng, H.P. The Effects of Acid Etching on the Nanomorphological Surface Characteristics and Activation Energy of Titanium Medical Materials. Materials 2017, 10, 1164. [Google Scholar] [CrossRef]
- Fu, L.; Omi, M.; Sun, M.; Cheng, B.; Mao, G.; Liu, T.; Mendonça, G.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Covalent Attachment of P15 Peptide to Ti Alloy Surface Modified with Polymer to Enhance Osseointegration of Implants. ACS Appl. Mater. Interfaces 2019, 11, 38531–38536. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, H.; Hong, S.; Yoon, S.J.; Kim, T.-H.; Lee, J.Y.; Hong, Y.T.; So, S. Surface-Initiated ATRP of Glycidyl Methacrylate in the Presence of Divinylbenzene on Porous Polystyrene-Based Resins for Boron Adsorption. Desalination 2020, 473, 114166–114173. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. Polydopamine-Melanin Initiators for Surface-Initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Fanton, L.; Cremasco, A.; Mello, M.G.; Caram, R. Anodization Growth of TiO2 Nanotubes on Ti–35Nb–7Zr–5Ta Alloy: Effects of Anodization Time, Strain Hardening, and Crystallographic Texture. J. Mater. Sci. 2019, 54, 13724–13739. [Google Scholar] [CrossRef]
- He, G.; Guo, B.; Wang, H.; Liang, C.; Ye, L.; Lin, Y.; Cai, X. Surface Characterization and Osteoblast Response to a Functionally Graded Hydroxyapatite/Fluoro-Hydroxyapatite/Titanium Oxide Coating on Titanium Surface by Sol-Gel Method. Cell Prolif. 2014, 47, 258–266. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Chen, K.; Wang, F.; Feng, C.; Xu, L.; Zhang, D. 3D Printed Chitosan-Gelatine Hydrogel Coating on Titanium Alloy Surface as Biological Fixation Interface of Artificial Joint Prosthesis. Int. J. Biol. Macromol. 2021, 182, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Chen, J.; Lai, M. Peptide GL13K Releasing Hydrogel Functionalized Micro/Nanostructured Titanium Enhances Its Osteogenic and Antibacterial Activity. J. Biomater. Sci. Polym. Ed. 2022, 34, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, A.J.; Park, J.E.; Jang, Y.S.; Lee, M.H. Antibacterial Activity and Biocompatibility with the Concentration of Ginger Fraction in Biodegradable Gelatin Methacryloyl (GelMA) Hydrogel Coating for Medical Implants. Polymers 2022, 14, 5317. [Google Scholar] [CrossRef] [PubMed]
- Gevrek, T.N.; Degirmenci, A.; Sanyal, R.; Sanyal, A. Multifunctional and Transformable ‘Clickable’ Hydrogel Coatings on Titanium Surfaces: From Protein Immobilization to Cellular Attachment. Polymers 2020, 12, 1211. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.; Gong, C.; Huang, J.; Xiong, D. A Biomimetic Bilayer Coating on Laser-Textured Ti6Al4V Alloy with Excellent Surface Wettability and Biotribological Properties for Artificial Joints. Ceram. Int. 2022, 48, 26264–26273. [Google Scholar] [CrossRef]
- Ji, S.; Li, X.; Wang, S.; Li, H.; Duan, H.; Yang, X.; Lv, P. Physically Entangled Antiswelling Hydrogels with High Stiffness. Macromol. Rapid Commun. 2022, 43, 2200272–2200282. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and Tough Hydrogel through Physical Cross-Linking and Molecular Alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef]
- Bilici, C.; Karaarslan, D.; Ide, S.; Okay, O. Toughness Improvement and Anisotropy in Semicrystalline Physical Hydrogels. Polymer 2018, 151, 208–217. [Google Scholar] [CrossRef]
- Croitoru, C.; Pop, M.A.; Bedo, T.; Cosnita, M.; Roata, I.C.; Hulka, I. Physically Crosslinked Poly(Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers 2020, 12, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Lai, X.; Ling, J.; Chen, D.; Liu, P.; Mao, T.; Shang, X.; Wang, L. Facile Preparation of Hydrogel Glue with High Strength and Antibacterial Activity from Physically Linked Network. Int. J. Pharm. 2022, 622, 121843–121852. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive Polysaccharides and Their Thermoreversible Physical Hydrogel Networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Monteiro, I.; Oliveira, A.S.; Nolasco, P.; Colaço, R.; Serro, A.P. Physically crosslinked polyvinyl alcohol hydrogels as synthetic cartilage materials. Ann. Med. 2021, 53, 25–26. [Google Scholar] [CrossRef]
- Tosakul, T.; Suetong, P.; Chanthot, P.; Pattamaprom, C. Degradation of Polylactic Acid and Polylactic Acid/Natural Rubber Blown Films in Aquatic Environment. J. Polym. Res. 2022, 29, 242–257. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Kureha, T.; Hayashi, K.; Li, X.; Shibayama, M. Mechanical Properties of Temperature-Responsive Gels Containing Ethylene Glycol in Their Side Chains. Soft Matter. 2020, 16, 10946–10953. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Q.; Yang, W.; Liu, S. Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion. Gels 2022, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, D.; Yuan, D.; He, C. Amphiphilic Conetworks and Gels Physically Cross-Linked via Stereocomplexation of Polylactide. Langmuir 2013, 29, 14307–14313. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, X.; Xiong, Z.; Sheng, D.; Lin, C.; Zhou, Y.; Yang, Y. Preparation of UV-Blocking Poly (vinylidene fluoride) Films through SI-AGET ATRP Using a Colorless Polydopamine Initiator Layer. Ind. Eng. Chem. Res. 2018, 57, 12662–12669. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.R.; Bian, X.C.; Cui, Y.; Li, G.; Chen, X.S. Investigation of poly(lactide) stereocomplexes: 3-Armed poly(L-lactide) blended with linear and 3-armed enantiomers. J. Phys. Chem. B 2012, 116, 9983–9991. [Google Scholar] [CrossRef]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, 1807101. [Google Scholar] [CrossRef]










| Sample | m(HEMA-PDLA29) (g) | m(HEMA-PLLA29) (g) | n(MEO2MA) (mmol) | n(OEGMA) (mmol) |
|---|---|---|---|---|
| PGel1 | 0.025 | 0.025 | 3.815 | 0.673 |
| PGel2 | 0.1 | 0.1 | 3.815 | 0.673 |
| PGel3 | 0.15 | 0.15 | 3.815 | 0.673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wu, Q.; Yang, W.; Wang, J.; Liu, Z.; Shi, H.; Liu, S. Preparation and Properties of Physical Gel on Medical Titanium Alloy Surface. Gels 2023, 9, 558. https://doi.org/10.3390/gels9070558
Fu Y, Wu Q, Yang W, Wang J, Liu Z, Shi H, Liu S. Preparation and Properties of Physical Gel on Medical Titanium Alloy Surface. Gels. 2023; 9(7):558. https://doi.org/10.3390/gels9070558
Chicago/Turabian StyleFu, Yu, Qingrong Wu, Wanying Yang, Jiaqi Wang, Zechen Liu, Hao Shi, and Shouxin Liu. 2023. "Preparation and Properties of Physical Gel on Medical Titanium Alloy Surface" Gels 9, no. 7: 558. https://doi.org/10.3390/gels9070558