Polysaccharide-Based Hydrogel from Seeds of Artemisia vulgaris: Extraction Optimization by Box–Behnken Design, pH-Responsiveness, and Sustained Drug Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. AVM Extraction
2.2. Effect of Factors Affecting the AVM Yield
2.2.1. pH
2.2.2. Temperature
2.2.3. S/W Ratio
2.2.4. S/W Time
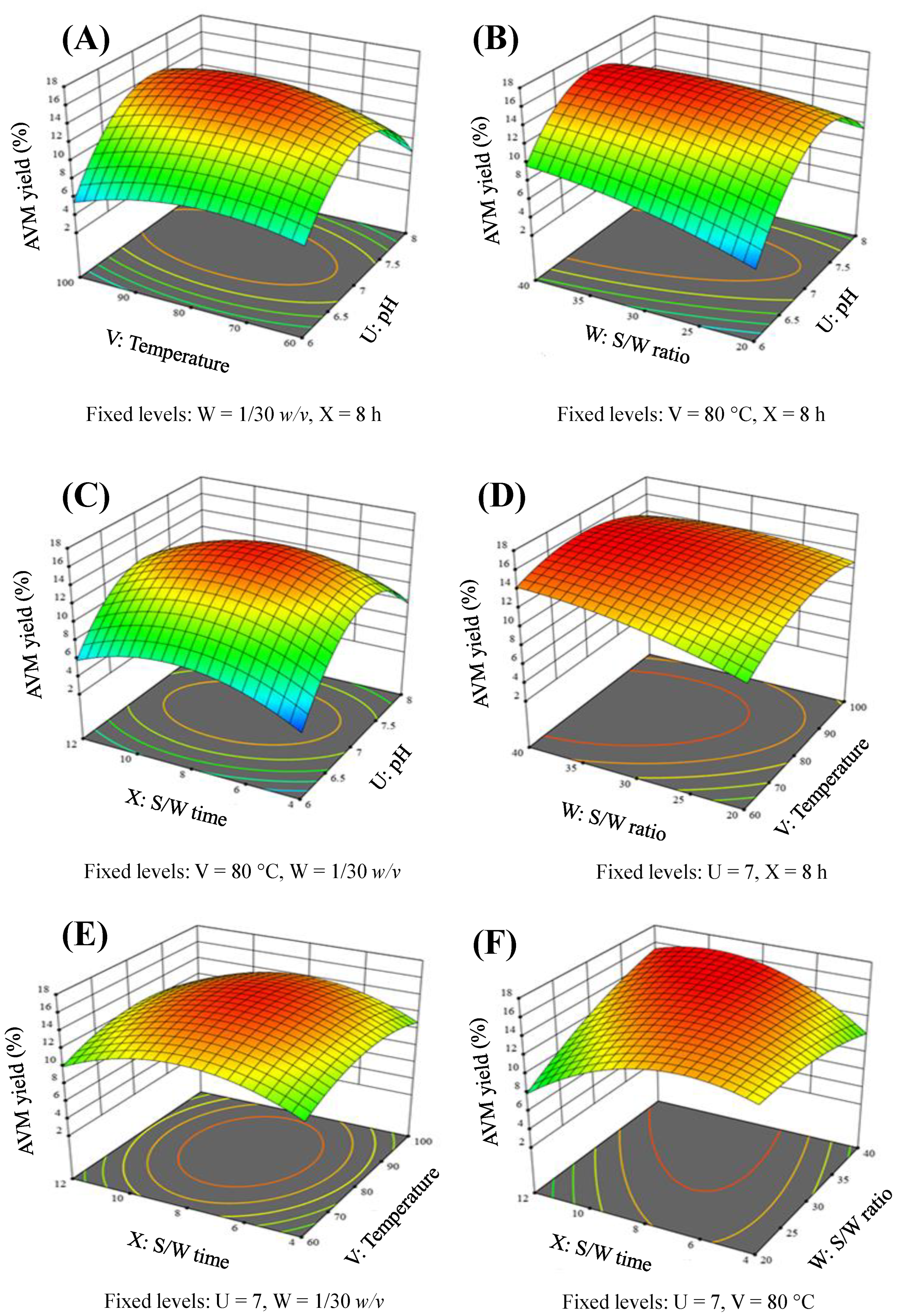
2.3. Response Surface Modeling
2.3.1. Interpretation of ANOVA
6.25U2 − 1.91V2 − 0.8517W2 − 3.10X2 + 1.23UV − 1.31UW − 0.7375UX −
0.8625VW + 0.0000VX + 2.43WX
2.3.2. Elucidation of Response Surface-Plots
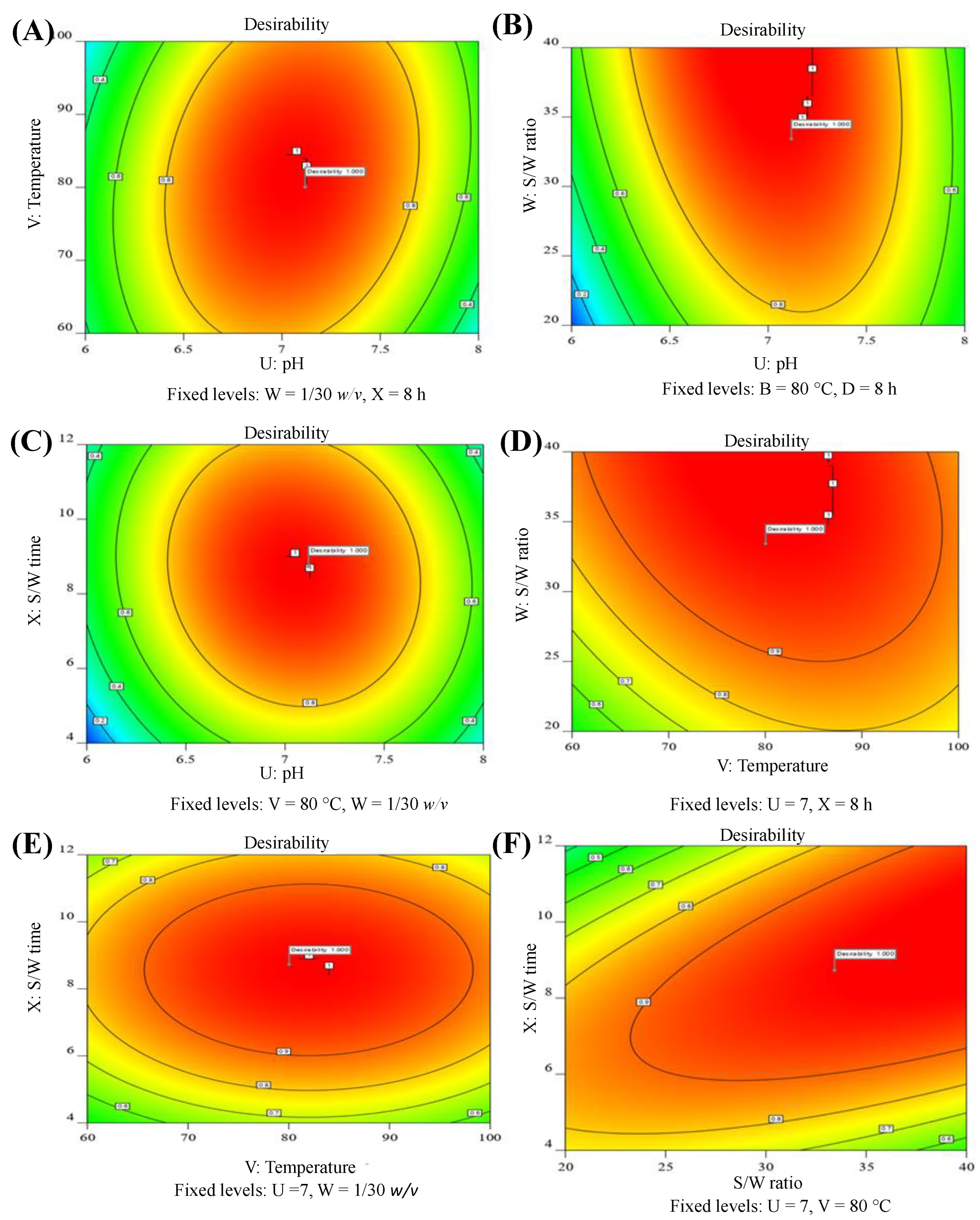
2.3.3. Checking of Model Adequacy and Desirability
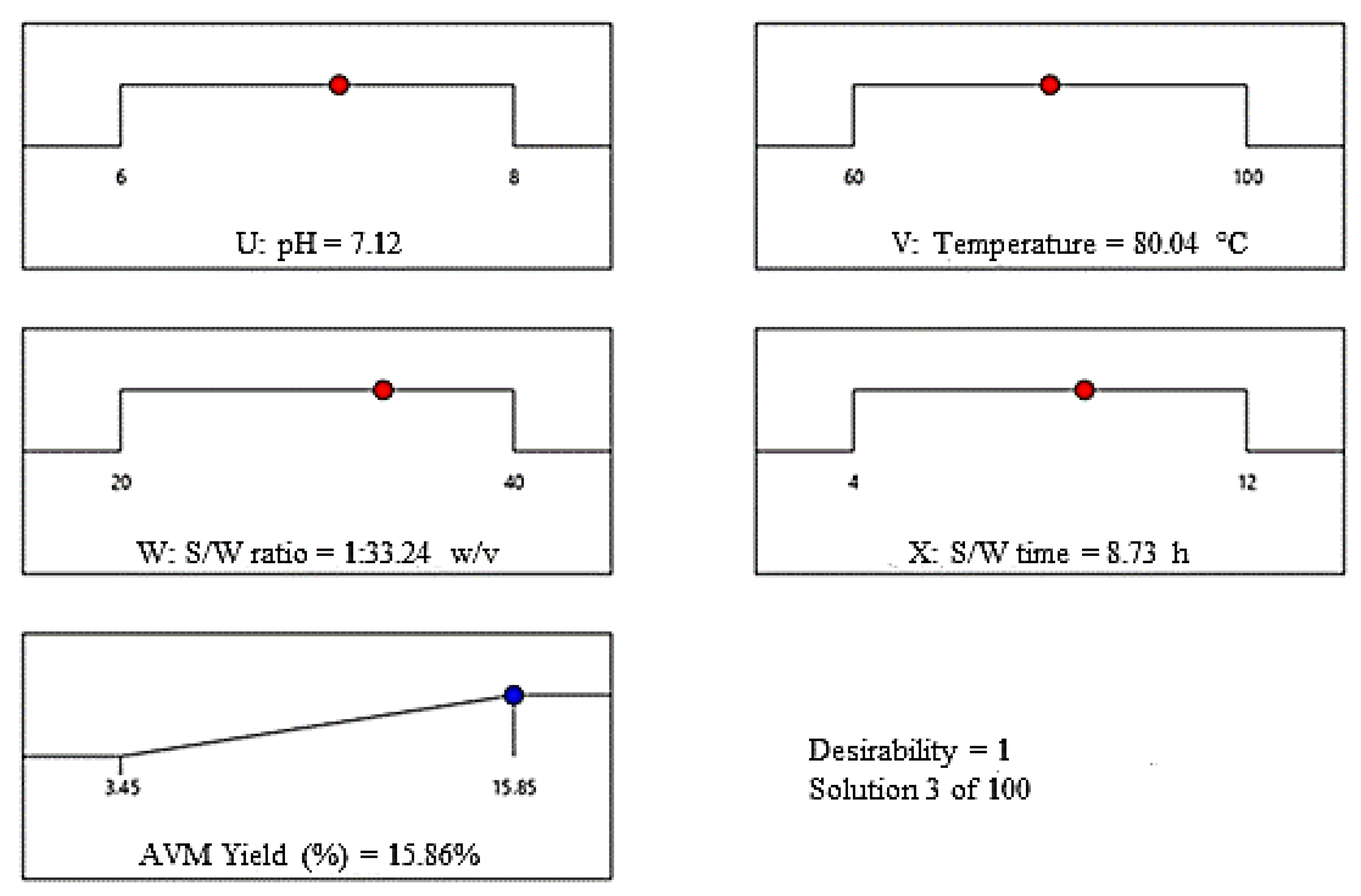
2.3.4. Optimum Conditions
2.3.5. Comparison of AVM Yield with the Yield of Already Reported Hydrogels
2.4. AVM as a Sustained Release Material
2.4.1. Compatibility Studies
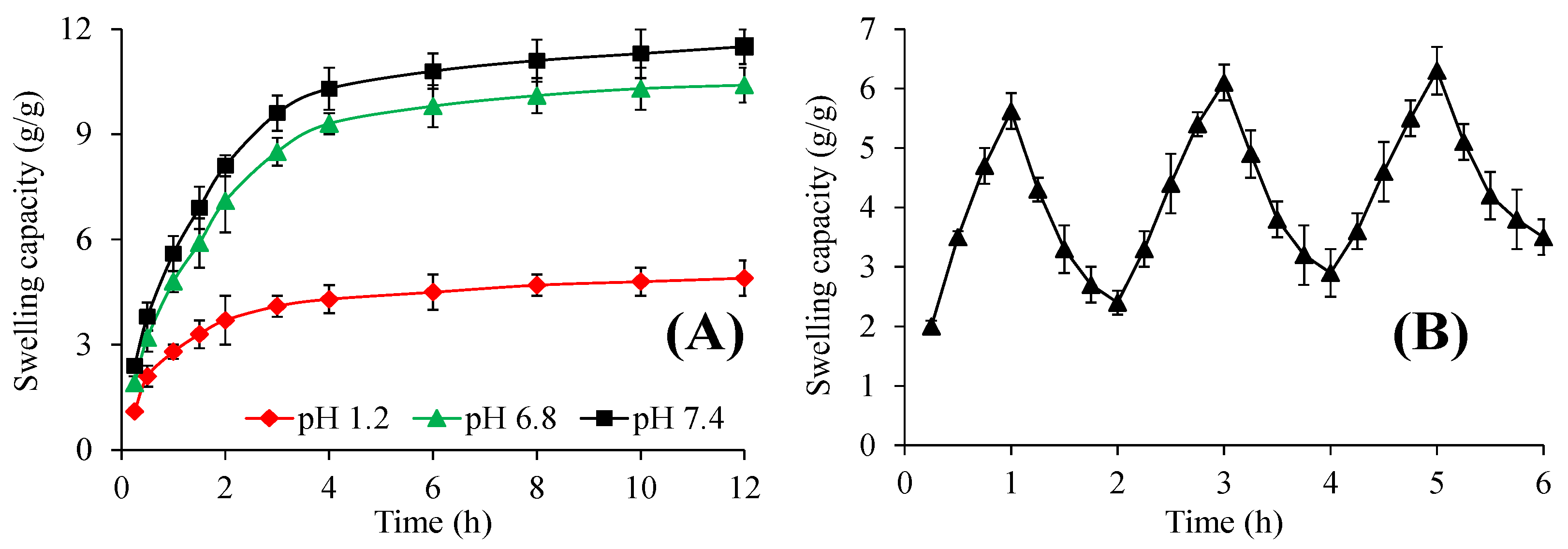
2.4.2. pH-Responsive Swelling
2.4.3. pH-Responsive Swelling/Deswelling
2.4.4. Esomeprazole Release and Release Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. AVM Extraction
4.2.2. Determination of Experimental Yield
4.2.3. Design of Experimentation and Statistical Validation
λ22V2 + λ33W2 + λ44X2 + λ1λ2UV + λ1λ3UW + λ1λ4UX + λ2λ3VW + λ2
λ4VX + λ3λ4WX + Ei
4.2.4. AVM as a Sustained Release Material
Preparation of AVM-Based Tablets
Compatibility Study
pH-Responsive Swelling
pH-Responsive Swelling/Deswelling
In Vitro Esomeprazole Release
Kinetics and Mechanism of Esomeprazole Release
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular characteristics and antioxidant activity of spruce (Picea abies) hemicelluloses isolated by catalytic oxidative delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, X.; Cheng, L.; Zhang, J.; Tang, L.; Tang, Y.; Kong, J.; Gu, J. Hybrid polymer membrane functionalized PBO fibers/cyanate esters wave-transparent laminated composites. Adv. Fiber Mater. 2022, 4, 520–531. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Junior, F.A.L.; Conceição, M.C.; de Resende, J.V.; Junqueira, L.A.; Pereira, C.G.; Prado, M.E.T. Response surface methodology for optimization of the mucilage extraction process from Pereskia aculeata Miller. Food Hydrocoll. 2013, 33, 38–47. [Google Scholar] [CrossRef]
- Ma, F.; Wang, R.; Li, X.; Kang, W.; Bell, A.E.; Zhao, D.; Liu, X.; Chen, W. Physical properties of mucilage polysaccharides from Dioscorea opposita Thunb. Food Chem. 2020, 311, 126039. [Google Scholar] [CrossRef]
- Wang, C.; Hou, Y.; Wang, X.; Li, W.; Zhang, Y.; Wang, S.; Zheng, J.; Hou, X. Structural and interfacial effects on drug release kinetics of liquid-based fibrous catheter. Adv. Fiber Mater. 2022, 4, 1645–1655. [Google Scholar] [CrossRef]
- Terra, D.A.; de Fátima, A.L.; de Almeida, C.M.T.J.; de Souza, F.A.; Santos-Filho, S.D.; Brandão-Neto, J. Effect of an extract of Artemisia vulgaris L. (mugwort) on the in vitro labeling of red blood cells and plasma proteins with technetium-99m. Braz. Arch. Biol. Technol. 2007, 50, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Uzun, E.; Sariyar, G.; Adsersen, A. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected plants. J. Ethnopharmacol. 2004, 95, 287–296. [Google Scholar] [CrossRef]
- Farid-ul-Haq, M.; Hussain, M.A.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Tabassum, T.; Hussain, I.; Sher, M.; Bukhari, S.N.A.; Naeem-ul-Hassan, M. A stimuli-responsive, superporous and non-toxic smart hydrogel from seeds of mugwort (Artemisia vulgaris): Stimuli responsive swelling/deswelling, intelligent drug delivery and enhanced aceclofenac bioavailability. RSC Adv. 2020, 10, 19832–19843. [Google Scholar] [CrossRef] [PubMed]
- Frasat, T.; Tulain, U.R.; Erum, A.; Saleem, U.; Sohail, M.F.; Kausar, R. Aloe vera and artemisia vulgaris hydrogels: Exploring the toxic effects of structural transformation of the biocompatible materials. Drug Dev. Ind. Pharm. 2021, 47, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Farid-ul-Haqa, M.; Alia, A.; Hussaina, M.A.; Abbasa, A.; Kausarb, F.; Aminc, H.M.; Shera, M.; Hussaine, S.Z.; Hussaine, I. Chemical modification of a polysaccharide from Artemisia vulgaris engenders a supersorbent for the removal of Cd2+ from spiked high-hardness groundwater. Desalin. Water Treat. 2021, 212, 129–142. [Google Scholar] [CrossRef]
- Sepúlveda, E.S.C.A.E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2017, 68, 534–545. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Bazezew, A.M.; Emire, S.A.; Sisay, M.T.; Teshome, P.G. Optimization of mucilage extraction from Ximenia americana seed using response surface methodology. Heliyon 2022, 8, e08781. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Haseeb, M.T.; Hussain, M.A.; Tayyab, M.; Muhammad, G.; Ahmad, N.; Alotaibi, N.F.; Hussain, S.Z.; Hussain, I. Extraction optimization of a superporous polysaccharide-based mucilage from Salvia spinosa L. Cell. Chem. Technol. 2022, 56, 957–969. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Laddha, H.; Gupta, R.; Agarwal, M. Non-toxic and biodegradable κ-carrageenan/ZnO hydrogel for adsorptive removal of norfloxacin: Optimization using response surface methodology. Int. J. Biol. Macromol. 2023, 238, 124145. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Ali, A.; Hussain, M.A.; Tayyab, M.; Alotaibi, N.F.; Elsherif, M.A.; Junaid, K.; Ejaz, H. Extraction optimization of mucilage from seeds of Mimosa pudica by response surface methodology. Polymers 2022, 14, 1904. [Google Scholar] [CrossRef]
- Golalikhani, M.; Khodaiyan, F.; Khosravi, A. Response surface optimization of mucilage aqueous extraction from flixweed (Descurainia sophia) seeds. Int. J. Biol. Macromol. 2014, 70, 444–449. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A.; Masoodi, F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017, 8, 235–244. [Google Scholar] [CrossRef]
- Bendahou, A.; Dufresne, A.; Kaddami, H.; Habibi, Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydr. Polym. 2007, 68, 601–608. [Google Scholar] [CrossRef]
- Amid, B.T.; Mirhosseini, H. Optimisation of aqueous extraction of gum from durian (Durio zibethinus) seed: A potential, low cost source of hydrocolloid. Food Chem. 2012, 132, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Siqueira dos Santos, S.; Bergamasco, R.; Antigo, J.; Madrona, G.S. Antioxidant activity, extraction and application of psyllium mucilage in chocolate drink. Nutr. Food Sci. 2010, 50, 1175–1185. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol. 2014, 66, 113–124. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Razavi, S.M.; Bostan, A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocol. 2009, 8, 2369–2379. [Google Scholar] [CrossRef]
- Hung, P.Y.; Lai, L.S. Structural characterization and rheological properties of the water extracted mucilage of Basella alba and the starch/aqueous mucilage blends. Food Hydrocoll. 2019, 93, 413–421. [Google Scholar] [CrossRef]
- Campos, B.E.; Ruivo, T.D.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.D.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Amin, A.M.; Ahmad, A.S.; Yin, Y.Y.; Yahya, N.; Ibrahim, N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocoll. 2007, 21, 273–279. [Google Scholar] [CrossRef]
- Singthong, J.; Ningsanond, S.; Cui, S.W. Extraction and physicochemical characterisation of polysaccharide gum from Yanang (Tiliacora triandra) leaves. Food Chem. 2009, 4, 1301–1307. [Google Scholar] [CrossRef]
- Farid-ul-Haq, M.; Haseeb, M.T.; Hussain, M.A.; Ashraf, M.U.; Naeem-ul-Hassan, M.; Hussain, S.Z.; Hussain, I. A smart drug delivery system based on Artemisia vulgaris hydrogel: Design, on-off switching, and real-time swelling, transit detection, and mechanistic studies. J. Drug Deliv. Sci. Technol. 2020, 58, 101795. [Google Scholar] [CrossRef]
- Pantić, M.; Kravanja, K.A.; Knez, Ž.; Novak, Z. Influence of the impregnation technique on the release of esomeprazole from various bioaerogels. Polymers 2021, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hussain, M.A.; Haseeb, M.T.; Bukhari, S.N.A.; Muhammad, G.; Sheikh, F.A.; Farid-ul-Haq, M.; Ahmad, N. A Smart Hydrogel from Salvia spinosa seeds: pH responsiveness, on-off switching, sustained drug release, and transit detection. Curr. Drug Deliv. 2023, 20, 292–305. [Google Scholar] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropylmethylcellulose. Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations- theoretical considerations and application to non-disintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef]






| Experiments | Tested Factors | Responses/Yields (%) | ||||
|---|---|---|---|---|---|---|
| U | V (°C) | W (w/v) | X (h) | Ya | Yp | |
| 1 | 7 | 80 | 30 | 8 | 15.75 | 15.48 |
| 2 | 8 | 80 | 30 | 12 | 6.21 | 6.65 |
| 3 | 8 | 80 | 20 | 8 | 9.56 | 9.57 |
| 4 | 7 | 100 | 30 | 12 | 11.25 | 11.13 |
| 5 | 8 | 80 | 30 | 4 | 7.67 | 7.86 |
| 6 | 8 | 60 | 30 | 8 | 6.9 | 6.69 |
| 7 | 7 | 100 | 30 | 4 | 10.76 | 10.87 |
| 8 | 7 | 80 | 40 | 4 | 9.95 | 10.21 |
| 9 | 6 | 80 | 20 | 8 | 3.45 | 4.69 |
| 10 | 6 | 80 | 30 | 12 | 6.21 | 5.88 |
| 11 | 7 | 60 | 30 | 12 | 9.62 | 10.08 |
| 12 | 7 | 80 | 30 | 8 | 15.65 | 15.48 |
| 13 | 7 | 100 | 20 | 8 | 12.75 | 12.87 |
| 14 | 7 | 60 | 30 | 4 | 9.13 | 9.82 |
| 15 | 7 | 60 | 20 | 8 | 10.11 | 10.09 |
| 16 | 6 | 60 | 30 | 8 | 7.56 | 6.90 |
| 17 | 7 | 80 | 20 | 12 | 8.67 | 7.98 |
| 18 | 7 | 100 | 40 | 8 | 13.75 | 13.63 |
| 19 | 7 | 80 | 20 | 4 | 13.25 | 12.59 |
| 20 | 7 | 80 | 30 | 8 | 14.43 | 15.48 |
| 21 | 7 | 80 | 30 | 8 | 15.85 | 15.48 |
| 22 | 8 | 100 | 30 | 8 | 9.97 | 10.20 |
| 23 | 8 | 80 | 40 | 8 | 10.11 | 9.44 |
| 24 | 7 | 80 | 40 | 12 | 15.11 | 15.34 |
| 25 | 6 | 80 | 30 | 4 | 4.72 | 4.14 |
| 26 | 6 | 80 | 40 | 8 | 9.25 | 9.81 |
| 27 | 7 | 80 | 30 | 8 | 15.72 | 15.48 |
| 28 | 7 | 60 | 40 | 8 | 14.56 | 14.30 |
| 29 | 6 | 100 | 30 | 8 | 5.72 | 5.50 |
| Source | SSE | DF | Mean | F-Values | p-Values |
|---|---|---|---|---|---|
| Model | 361.82 | 14 | 25.84 | 53.05 | <0.0001 ss |
| Linear terms | |||||
| U-pH | 15.21 | 1 | 15.21 | 31.22 | <0.0001 ss |
| V-Temperature (°C) | 3.33 | 1 | 3.33 | 6.83 | 0.0204 s |
| W-S/W ratio (w/v) | 18.60 | 1 | 18.60 | 38.18 | <0.0001 ss |
| X-S/W time (h) | 0.2107 | 1 | 0.2107 | 0.4325 | 0.5215 ns |
| Quadratic terms | |||||
| U2 | 253.41 | 1 | 253.41 | 520.18 | <0.0001 ss |
| V2 | 23.58 | 1 | 23.58 | 48.40 | <0.0001 ss |
| W2 | 4.70 | 1 | 4.70 | 9.66 | 0.0077 hs |
| X2 | 62.25 | 1 | 62.25 | 127.78 | <0.0001 ss |
| Interaction terms | |||||
| UV | 6.03 | 1 | 6.03 | 12.37 | 0.0034 hs |
| UW | 6.89 | 1 | 6.89 | 14.14 | 0.0021 hs |
| UX | 2.18 | 1 | 2.18 | 4.47 | 0.0530 ns |
| VW | 2.98 | 1 | 2.98 | 6.11 | 0.0269 s |
| VX | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 ns |
| WX | 23.72 | 1 | 23.72 | 48.68 | <0.0001 ss |
| Residual | 6.82 | 14 | 0.4872 | ||
| Cor total | 368.64 | 28 | |||
| Lack of fit | 5.42 | 10 | 0.5421 | 1.55 | 0.3572 ns |
| Pure error | 1.40 | 4 | 0.3497 | ||
| R2 = 0.9815; R2-adjusted = 0.9630; R2-predicted = 0.9094; ±SD = 0.6980; %CV = 6.67%; Mean = 10.47; ADP = 22.59; Predicted error sum of square (PRESS) = 33.41 | |||||
| Botanical Name of Plants | Common Name of Plants | Source of Hydrogel | Yield (%) | References |
|---|---|---|---|---|
| Durio zibethinus | Durian | Seeds | 1.2 | [28] |
| Tiliacora triandra | Yanang gum | Leaves | 4.54 | [29] |
| Salvia hispanica | Chia | Seeds | 4.95 | [27] |
| Lepidium perfoliatum | Cress | seeds | 6.46 | [25] |
| Salvia spinosa | Kannocha | Seeds | 7.35 | [16] |
| Descurainia sophia | Flixweed | seeds | 10.45 | [19] |
| Mimosa pudica | Touch-me-not | Seeds | 10.66 | [18] |
| Artemisia vulgaris | Mugwort | Seeds | 15.86 (Highest) | Present study |
| Tablet | Zero-Order Kinetic Model | Korsmeyer-Peppas Model | |||
|---|---|---|---|---|---|
| AVT3 | R2 0.9942 | K0 7.97 | R2 | KKP | n |
| 0.9979 | 18.3780 | 0.471 | |||
| Composition of the Tablets | AVT1 | AVT2 | AVT3 |
|---|---|---|---|
| AVM | 150 | 200 | 250 |
| Esomeprazole | 80 | 80 | 80 |
| Tragacanth gum | 60 | 60 | 60 |
| MC-cellulose | 100 | 50 | - |
| Mg-stearate | 10 | 10 | 10 |
| Total weight | 400 | 400 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.A.; Ali, A.; Alsahli, T.G.; Khan, N.; Sharif, A.; Haseeb, M.T.; Alsaidan, O.A.; Tayyab, M.; Bukhari, S.N.A. Polysaccharide-Based Hydrogel from Seeds of Artemisia vulgaris: Extraction Optimization by Box–Behnken Design, pH-Responsiveness, and Sustained Drug Release. Gels 2023, 9, 525. https://doi.org/10.3390/gels9070525
Hussain MA, Ali A, Alsahli TG, Khan N, Sharif A, Haseeb MT, Alsaidan OA, Tayyab M, Bukhari SNA. Polysaccharide-Based Hydrogel from Seeds of Artemisia vulgaris: Extraction Optimization by Box–Behnken Design, pH-Responsiveness, and Sustained Drug Release. Gels. 2023; 9(7):525. https://doi.org/10.3390/gels9070525
Chicago/Turabian StyleHussain, Muhammad Ajaz, Arshad Ali, Tariq G. Alsahli, Nadia Khan, Ahsan Sharif, Muhammad Tahir Haseeb, Omar Awad Alsaidan, Muhammad Tayyab, and Syed Nasir Abbas Bukhari. 2023. "Polysaccharide-Based Hydrogel from Seeds of Artemisia vulgaris: Extraction Optimization by Box–Behnken Design, pH-Responsiveness, and Sustained Drug Release" Gels 9, no. 7: 525. https://doi.org/10.3390/gels9070525







