Physical, Chemical, Barrier, and Antioxidant Properties of Pectin/Collagen Hydrogel-Based Films Enriched with Melissa officinalis
Abstract
:1. Introduction
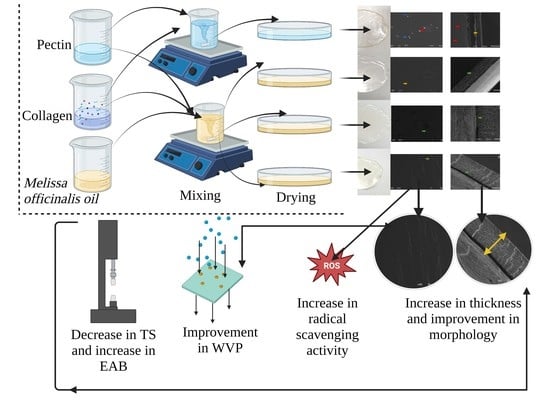
2. Results and Discussion
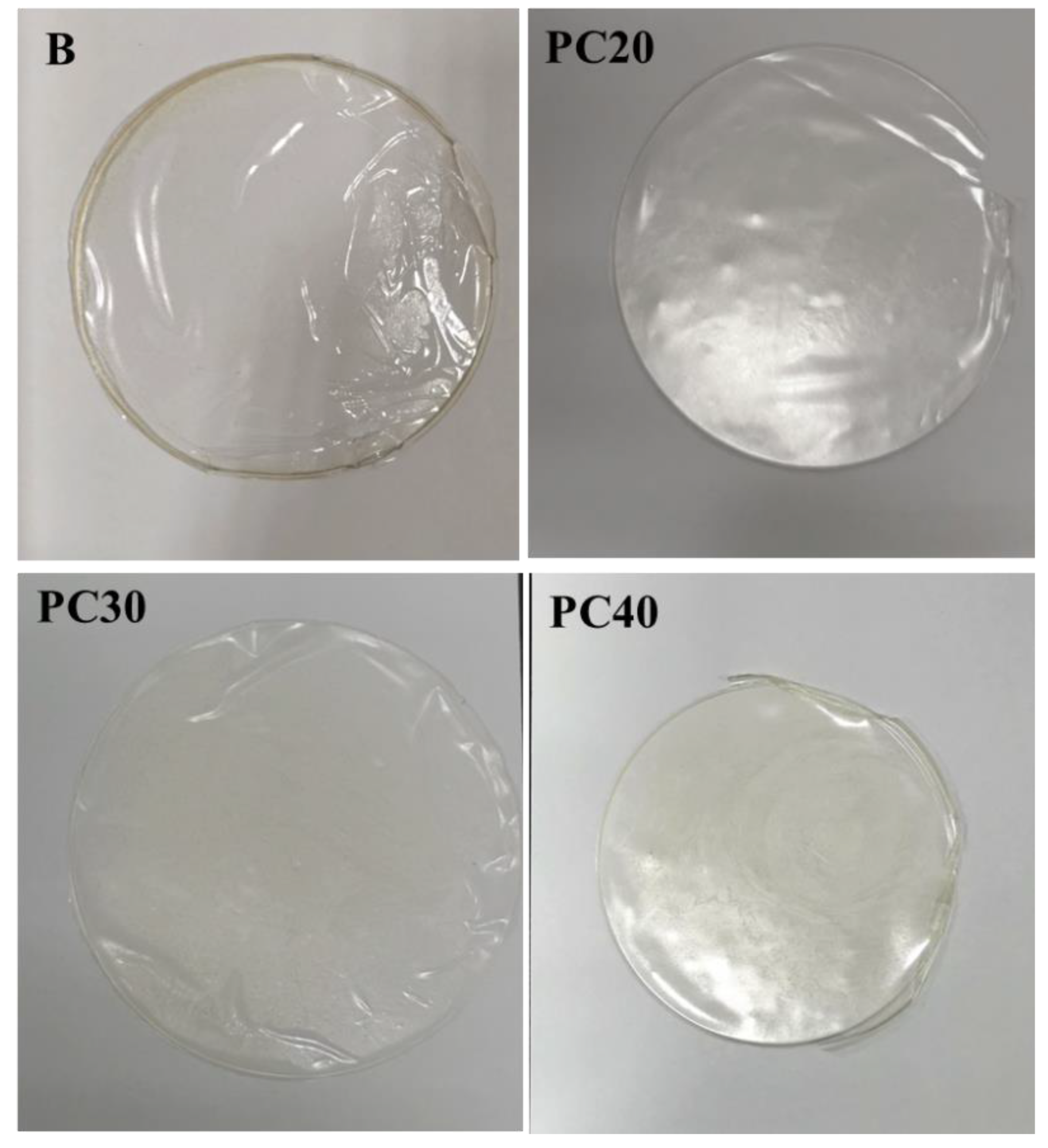
2.1. Visual Appearance
2.2. GC-MS Analysis of Melissa Essential Oil
2.3. Thickness
2.4. Mechanical Attributes
2.5. Barrier Properties
2.6. Transparency and Color Analysis
2.7. Scanning Electron Microscopy (SEM)
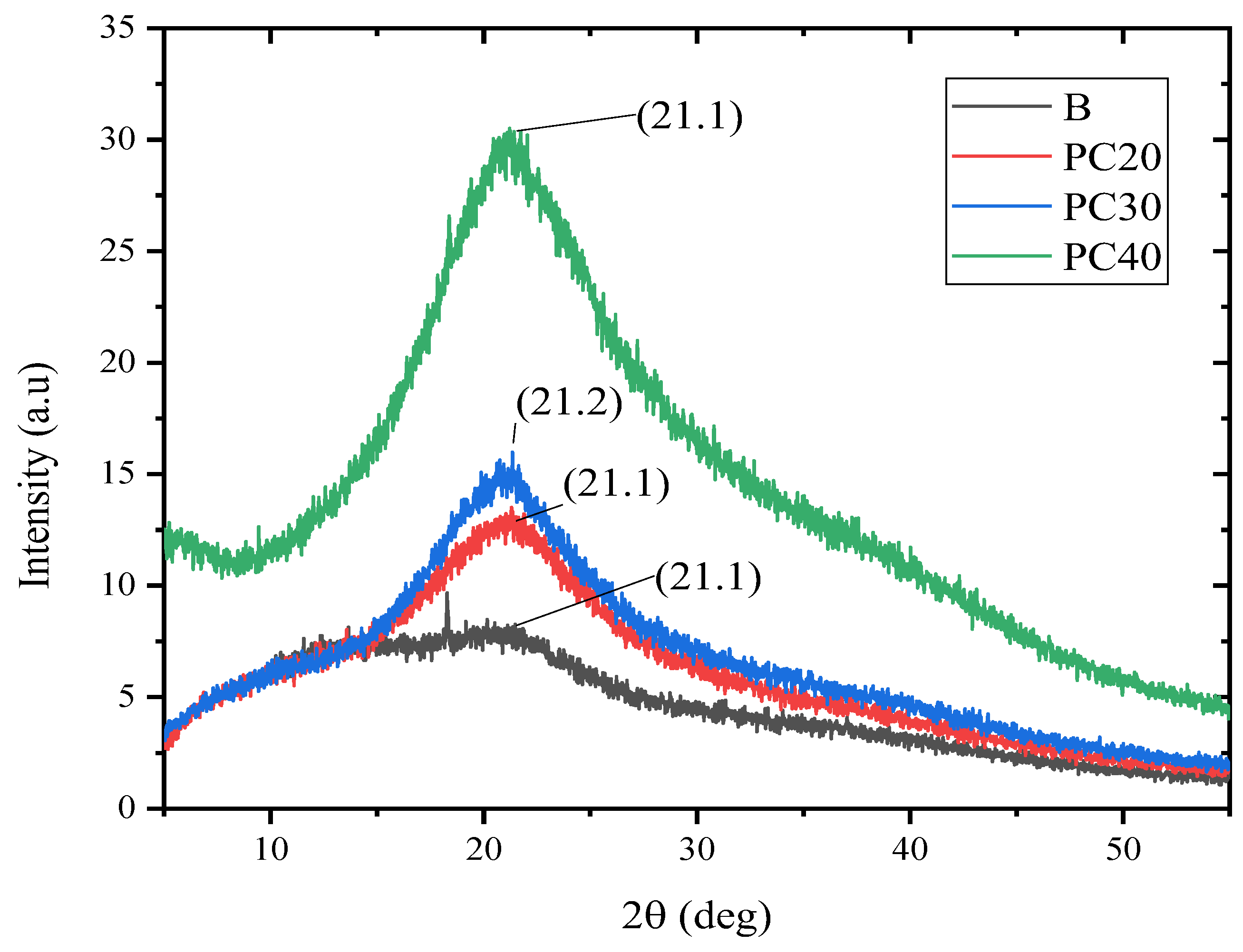
2.8. X-ray Diffraction (XRD) Analysis
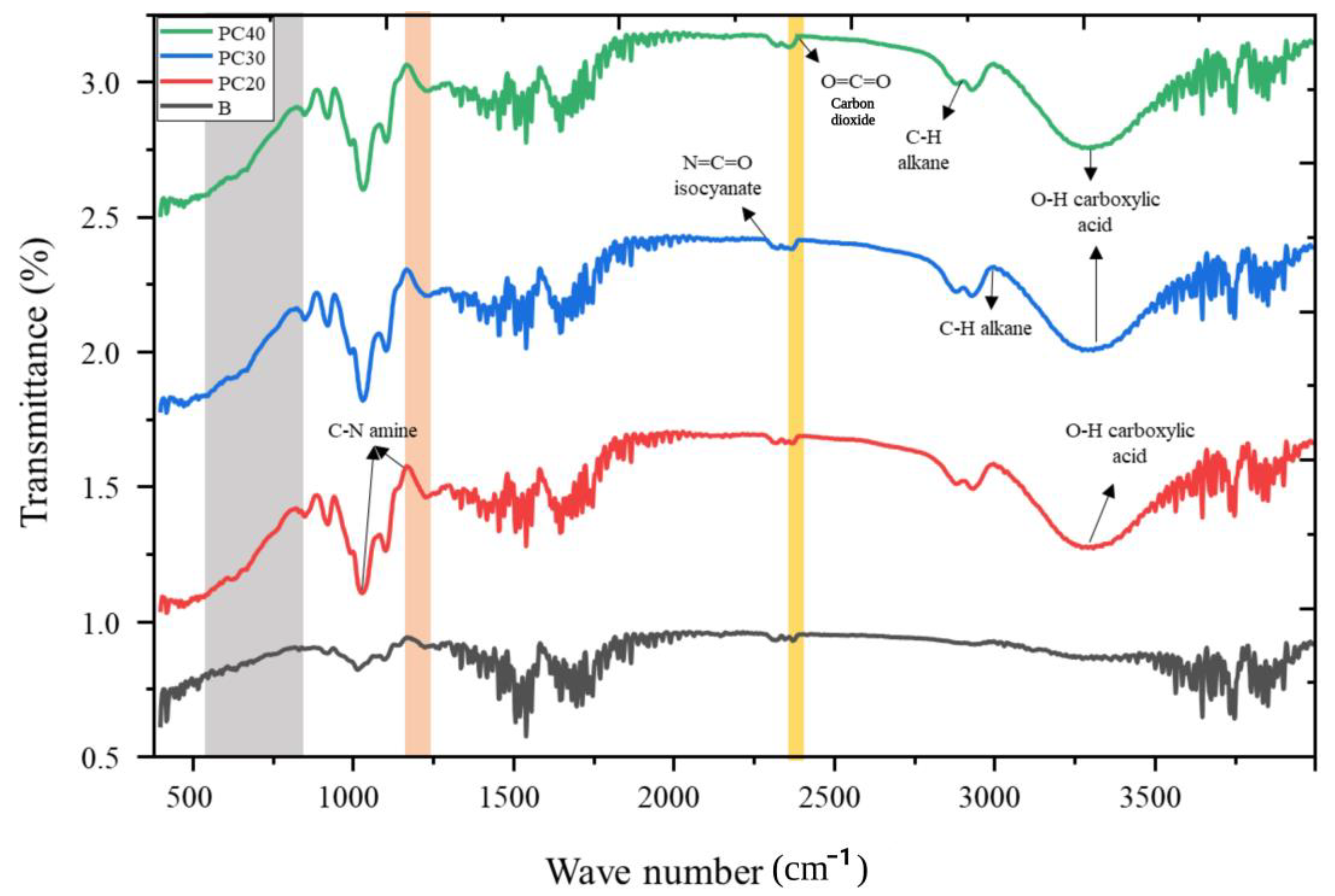
2.9. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
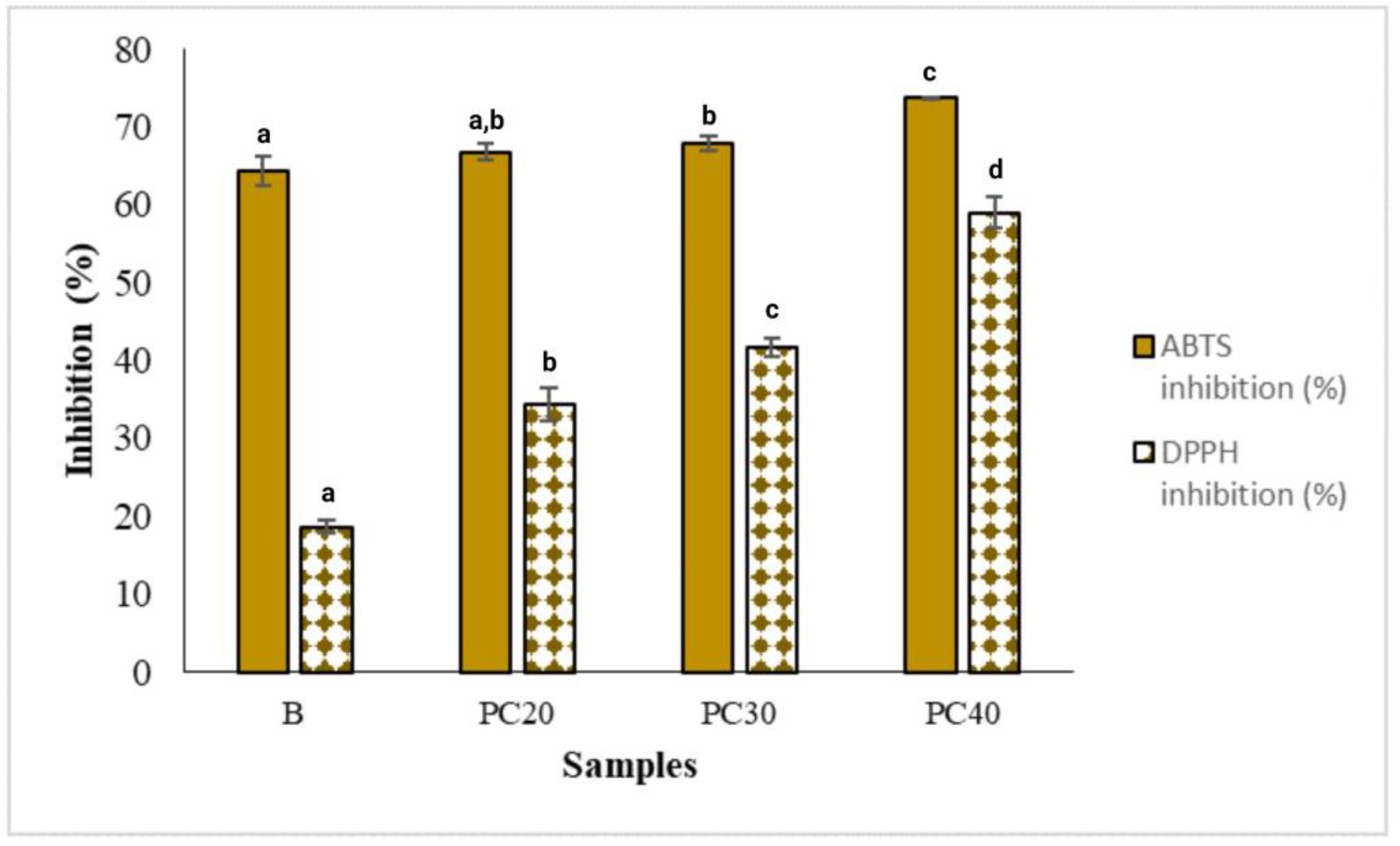
2.10. Antioxidant Activities of the Hydrogel-Based Films
3. Conclusions
4. Materials and Methods
4.1. Material Procurement
4.2. Film Preparation
4.3. Chromatographic (GC/MS) Analysis
4.4. The Thickness of the Films
4.5. Mechanical Testing
4.6. Water Vapor Permeability (WVP)
4.7. Moisture Content (MC)
4.8. Transparency and Color Analysis
4.9. Scanning Electron Microscopy (SEM)
4.10. X-ray Diffraction (XRD) Analysis
4.11. FTIR Spectra Analysis
4.12. Antioxidant Activity
4.13. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Morais Lima, M.; Carneiro, L.C.; Bianchini, D.; Dias, A.R.G.; Zavareze, E.d.R.; Prentice, C.; Moreira, A.d.S. Structural, thermal, physical, mechanical, and barrier properties of chitosan films with the addition of xanthan gum. J. Food Sci. 2017, 82, 698–705. [Google Scholar] [CrossRef]
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; González, L.-M. Process development and simulation of pectin extraction from orange peels. Food Bioprod. Process. 2015, 96, 86–98. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.; Ortega-Ramirez, L.; Cruz-Valenzuela, M.; Silva-Espinoza, B.; Gonzalez-Aguilar, G.; Ayala-Zavala, J. Combinational Approaches for Antimicrobial Packaging: Pectin and Cinnamon Leaf Oil. Antimicrob. Food Packag. 2016, 609–617. [Google Scholar] [CrossRef]
- Borges, J.G.; Silva, A.G.; Cervi-Bitencourt, C.; Vanin, F.M.; Carvalho, R.A.d. Lecithin, gelatin and hydrolyzed collagen orally disintegrating films: Functional properties. Int. J. Biol. Macromol. 2016, 86, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, S.; Wang, H. Scale-up preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017, 2017, 4954259. [Google Scholar]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Ali, A.; Noh, N.M.; Mustafa, M.A. Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag. Shelf Life 2015, 3, 56–61. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Chafer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Santos-Neto, L.L.d.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; de Souza, G.A. The use of herbal medicine in Alzheimer’s disease—A systematic review. Evid.-Based Complement. Altern. Med. 2006, 3, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Karimi Sani, I.; Alizadeh, M.; Pirsa, S.; Moghaddas Kia, E. Impact of operating parameters and wall material components on the characteristics of microencapsulated Melissa officinalis essential oil. Flavour Fragr. J. 2019, 34, 104–112. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, C.; Xie, Y.; Mei, J.; Xie, J. Effect of Melissa officinalis L. essential oil nanoemulsions on structure and properties of carboxymethyl chitosan/locust bean gum composite films. Membranes 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.K.; Marand, S.A.; Alizadeh, M.; Amiri, S.; Asdagh, A. Thermal, mechanical, microstructural and inhibitory characteristics of sodium caseinate based bioactive films reinforced by ZnONPs/encapsulated Melissa officinalis essential oil. J. Inorg. Organomet. Polym. Mater. 2021, 31, 261–271. [Google Scholar] [CrossRef]
- Sorensen, J.M. Melissa officinalis. Int. J. Aromather. 2000, 10, 7–15. [Google Scholar] [CrossRef]
- Mrlianova, M.; Tekel’ova, D.; Felklova, M.; Toth, J.; Musil, P.; Grancai, D. Comparison of the quality of Melissa officinalis L. cultivar Citra with Mellissas of European origin. Ceska A Slov. Farm. Cas. Ceske Farm. Spol. Slov. Farm. Spol. 2001, 50, 299–302. [Google Scholar]
- Park, J.-H.; Lee, H.-S. Acaricidal target and mite indicator as color alteration using 3,7-dimethyl-2,6-octadienal and its derivatives derived from Melissa officinalis leaves. Sci. Rep. 2018, 8, 8129. [Google Scholar] [CrossRef] [Green Version]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1934578X0900400827. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and characterization of chitosan and porphyran based composite edible films containing ginger essential oil. Polymers 2022, 14, 1782. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q. Development and characterization of novel antimicrobial bilayer films based on Polylactic acid (PLA)/Pickering emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Scartazzini, L.; Tosati, J.; Cortez, D.; Rossi, M.; Flôres, S.; Hubinger, M.; Di Luccio, M.; Monteiro, A. Gelatin edible coatings with mint essential oil (Mentha arvensis): Film characterization and antifungal properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef] [PubMed]
- Behjati, J.; Yazdanpanah, S. Nanoemulsion and emulsion vitamin D3 fortified edible film based on quince seed gum. Carbohydr. Polym. 2021, 262, 117948. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels 2023, 9, 233. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Jawad, M.; Shah, Y.A.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. A Comparative Study of the Properties of Gelatin (Porcine and Bovine)-Based Edible Films Loaded with Spearmint Essential Oil. Biomimetics 2023, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002.
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioprod. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, C. Film Transparency and Opacity Measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]


| No. | NAME | R. Time | Composition % |
|---|---|---|---|
| 1 | 2,6-OCTADIENAL,3,7-DIMETHYL | 20.005 | 20.40 |
| 2 | Citral | 21.495 | 19.64 |
| 3 | Caryophyllene | 27.183 | 9.28 |
| 4 | Geranyl acetate | 25.788 | 7.33 |
| 5 | Caryophyllene oxide | 33.622 | 6.85 |
| 6 | Citronellal | 15.68 | 6.58 |
| 7 | Linalool | 13.413 | 6.00 |
| Film Samples | Thickness (mm) | Elongation at Break (%) | Tensile Strength (MPa) | Water Vapor Permeability (g mm/m2 h kPa) | Moisture Content (%) |
|---|---|---|---|---|---|
| B | 0.061 ± 0.005 a | 27.36 ± 1.94 a | 8.12 ± 0.68 a | 0.676 ± 0.02 a | 29.92 ± 0.53 a |
| PC20 | 0.068 ± 0.005 ab | 30.24 ± 2.23 bc | 3.48 ± 0.11 b | 0.569 ± 0.04 b | 28.30 ± 0.57 b |
| PC30 | 0.072 ± 0.008 b | 34.97 ± 3.76 cd | 2.37 ± 0.21 c | 0.536± 0.05 b | 26.55 ± 0.23 c |
| PC40 | 0.073 ± 0.006 b | 36.29 ± 2.80 d | 1.25 ± 0.16 d | 0.327 ± 0.01 c | 25.16 ± 0.83 c |
| Film Samples | Transparency (%) | L | a* | b* | ΔE* |
|---|---|---|---|---|---|
| B | 90.37 ± 0.55 a | 95.82 ± 0.05 a | −0.10 ± 0.01 a | 0.95 ± 0.07 a | 0.85 ± 0.07 a |
| PC20 | 87.30 ± 0.28 b | 96.07 ± 0.02 a | −0.15 ± 0.02 b | 1.32 ± 0.13 b | 1.16 ± 0.08 b |
| PC30 | 84.33 ± 0.55 c | 96.52 ± 0.34 a | −0.17 ± 0.01 b | 1.57 ± 0.27 bc | 1.63 ± 0.01 c |
| PC40 | 82.80 ± 0.75 c | 96.54 ± 0.46 a | −0.23 ± 0.02 c | 1.57 ± 0.06 c | 1.90 ± 0.01 d |
| Film Samples | Film Forming Components |
|---|---|
| B/Control | (1.5%) Pectin (w/v) + 1% Collagen (w/v) + (1.25%) Glycerol (v/v) |
| PC20 | (1.5%) Pectin (w/v) + 1% Collagen (w/v) + (1.25%) Glycerol (v/v) + (0.2%) Tween 80 (v/v) + (0.1%) MOEO (v/v) |
| PC30 | (1.5%) Pectin (w/v) + 1% Collagen (w/v) + (1.25%) Glycerol (v/v) + (0.3%) Tween 80 (v/v) + (0.15%) MOEO (v/v) |
| PC40 | (1.5%) Pectin (w/v) + 1% Collagen (w/v) + (1.25%) Glycerol (v/v) + (0.4%) Tween 80 (v/v) + (0.2%) MOEO (v/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, S.; Al-Harrasi, A.; Alhadhrami, A.S.; Shah, Y.A.; Kotta, S.; Iqbal, J.; Anwer, M.K.; Nair, A.K.; Koca, E.; Aydemir, L.Y. Physical, Chemical, Barrier, and Antioxidant Properties of Pectin/Collagen Hydrogel-Based Films Enriched with Melissa officinalis. Gels 2023, 9, 511. https://doi.org/10.3390/gels9070511
Bhatia S, Al-Harrasi A, Alhadhrami AS, Shah YA, Kotta S, Iqbal J, Anwer MK, Nair AK, Koca E, Aydemir LY. Physical, Chemical, Barrier, and Antioxidant Properties of Pectin/Collagen Hydrogel-Based Films Enriched with Melissa officinalis. Gels. 2023; 9(7):511. https://doi.org/10.3390/gels9070511
Chicago/Turabian StyleBhatia, Saurabh, Ahmed Al-Harrasi, Aysha Salim Alhadhrami, Yasir Abbas Shah, Sabna Kotta, Javed Iqbal, Md Khalid Anwer, Anjana Karunakaran Nair, Esra Koca, and Levent Yurdaer Aydemir. 2023. "Physical, Chemical, Barrier, and Antioxidant Properties of Pectin/Collagen Hydrogel-Based Films Enriched with Melissa officinalis" Gels 9, no. 7: 511. https://doi.org/10.3390/gels9070511