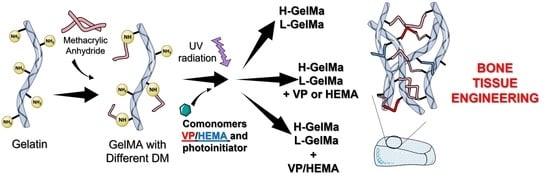
New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
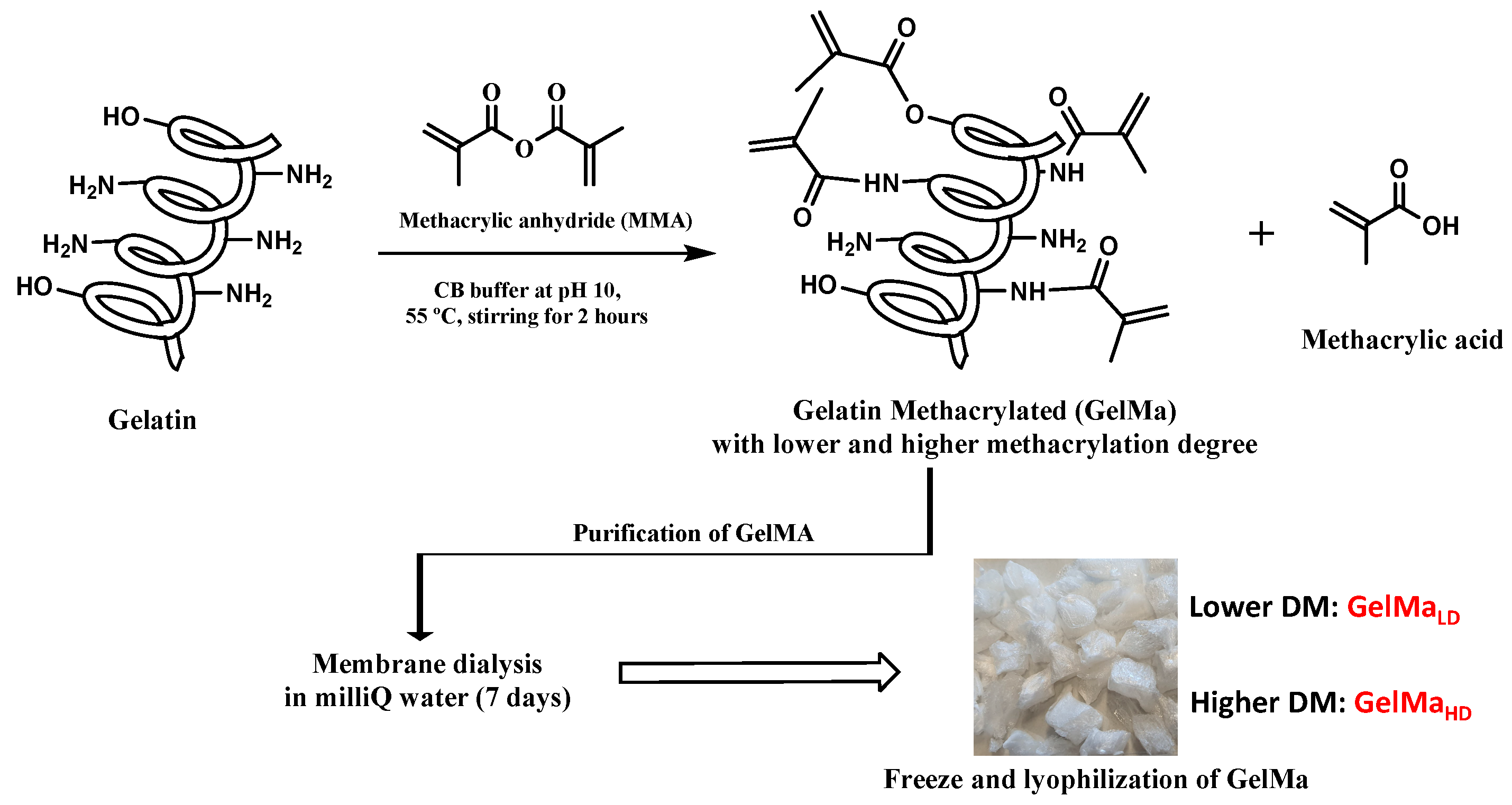
2.1. Synthesis of GelMa with Two Methacryloylation Degrees (DM)
2.2. Biomaterial Preparation and Characterization GelMa and GelMa Copolymers
2.2.1. Biopolymer Preparation
- Why this strategy?
- Biopolymer synthesis
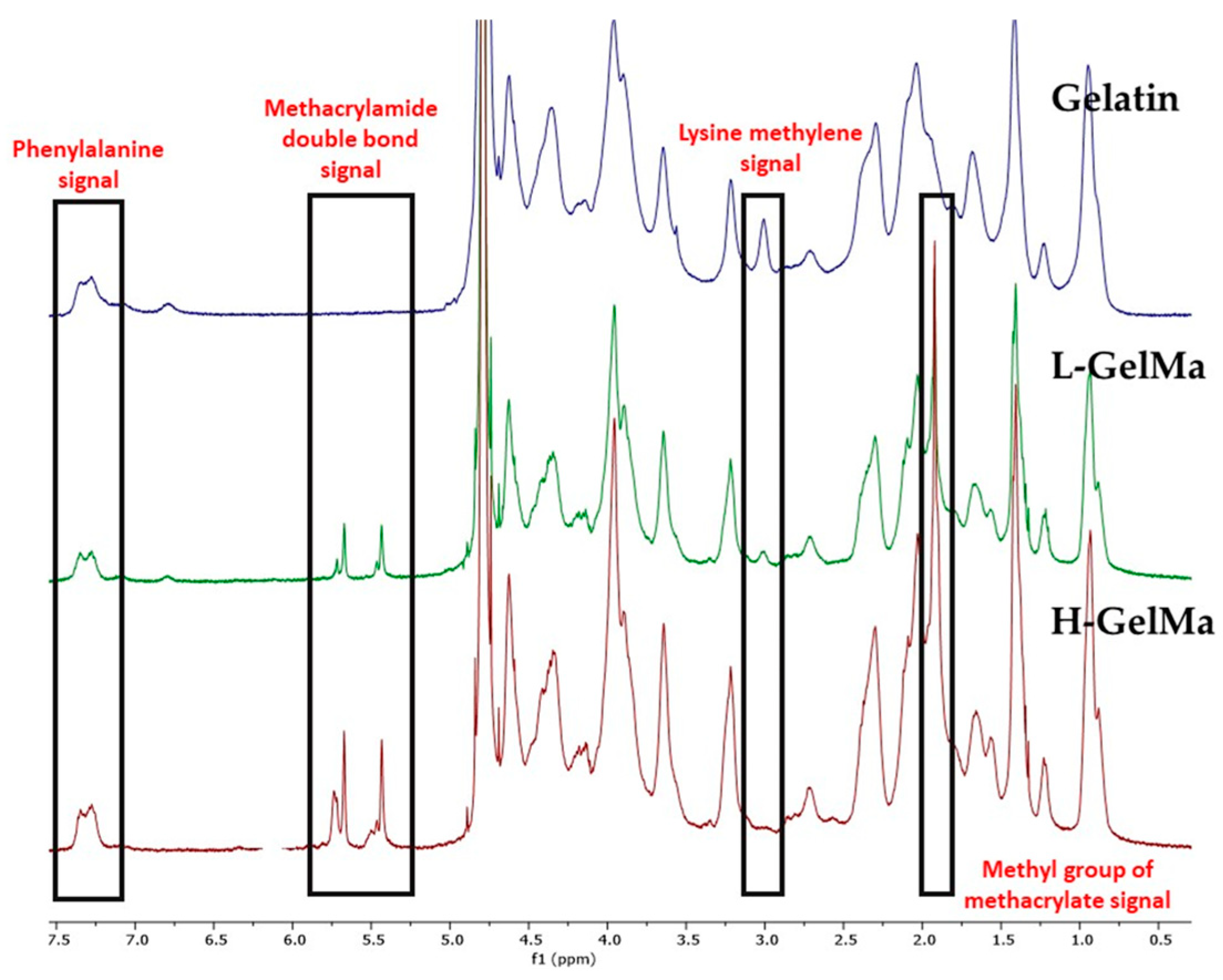
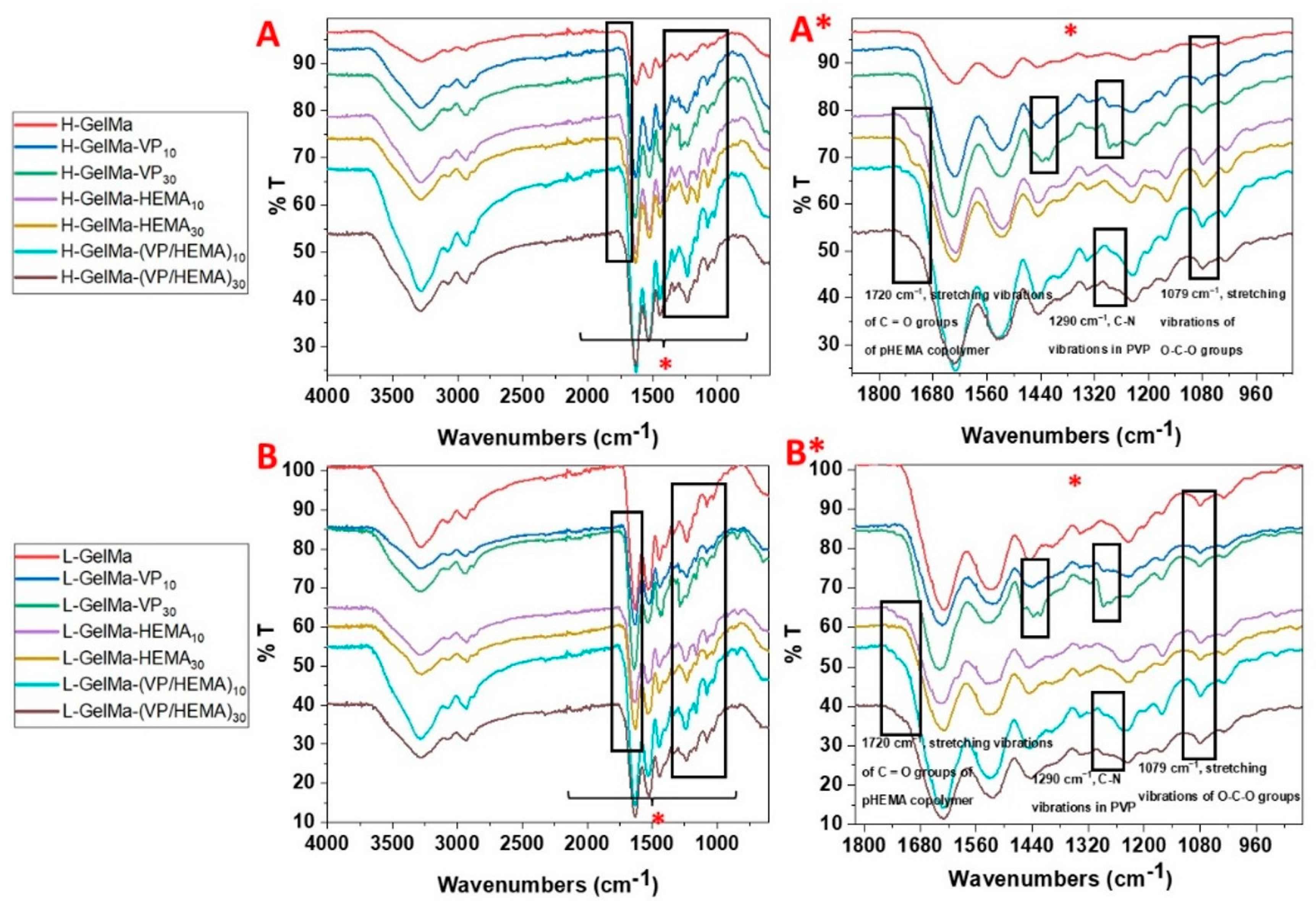
2.2.2. Biopolymer Characterization
- FTIR spectroscopy
- Thermal properties: TGA analysis
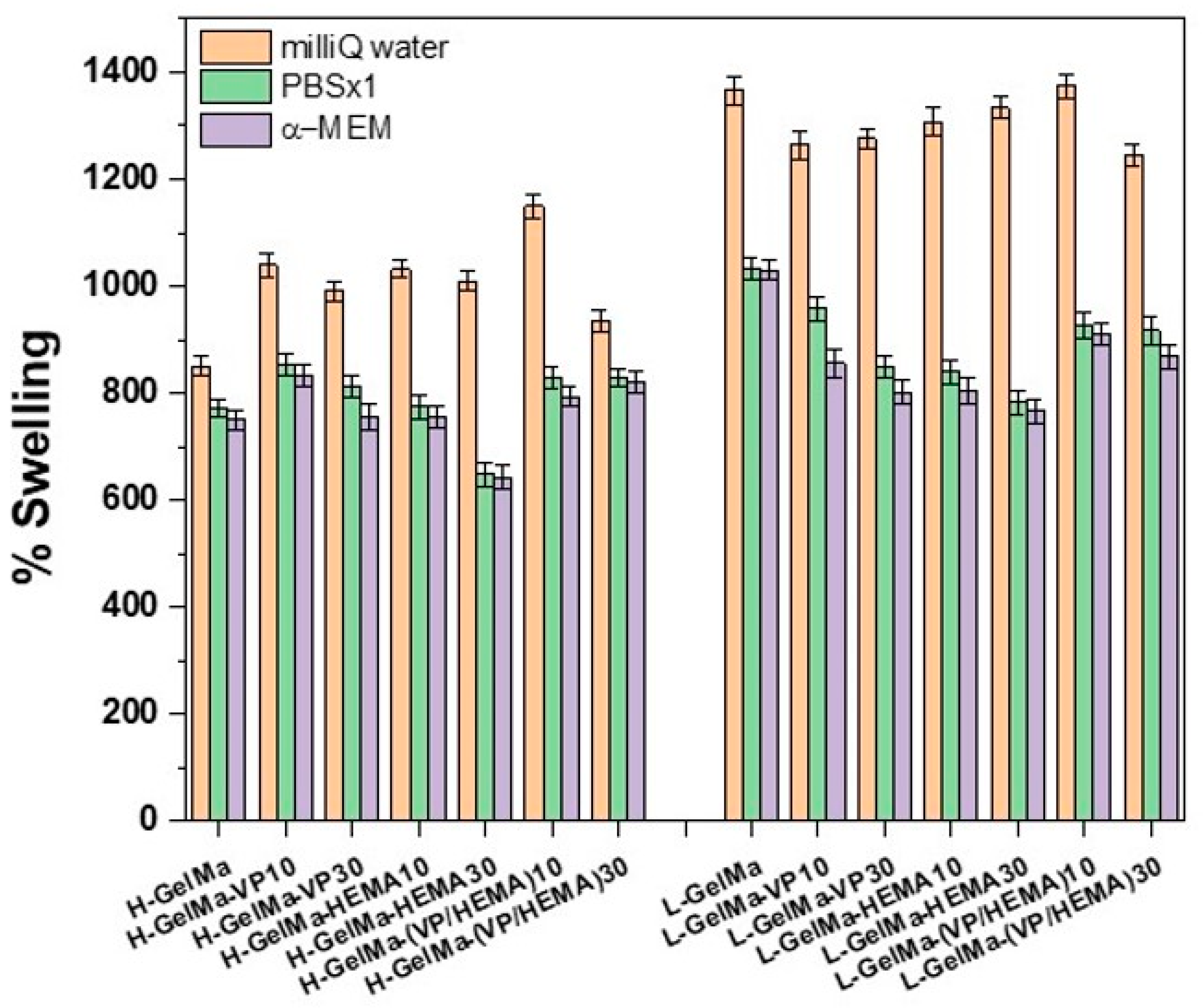
- Swelling behavior
- Determination of porosity by SEM
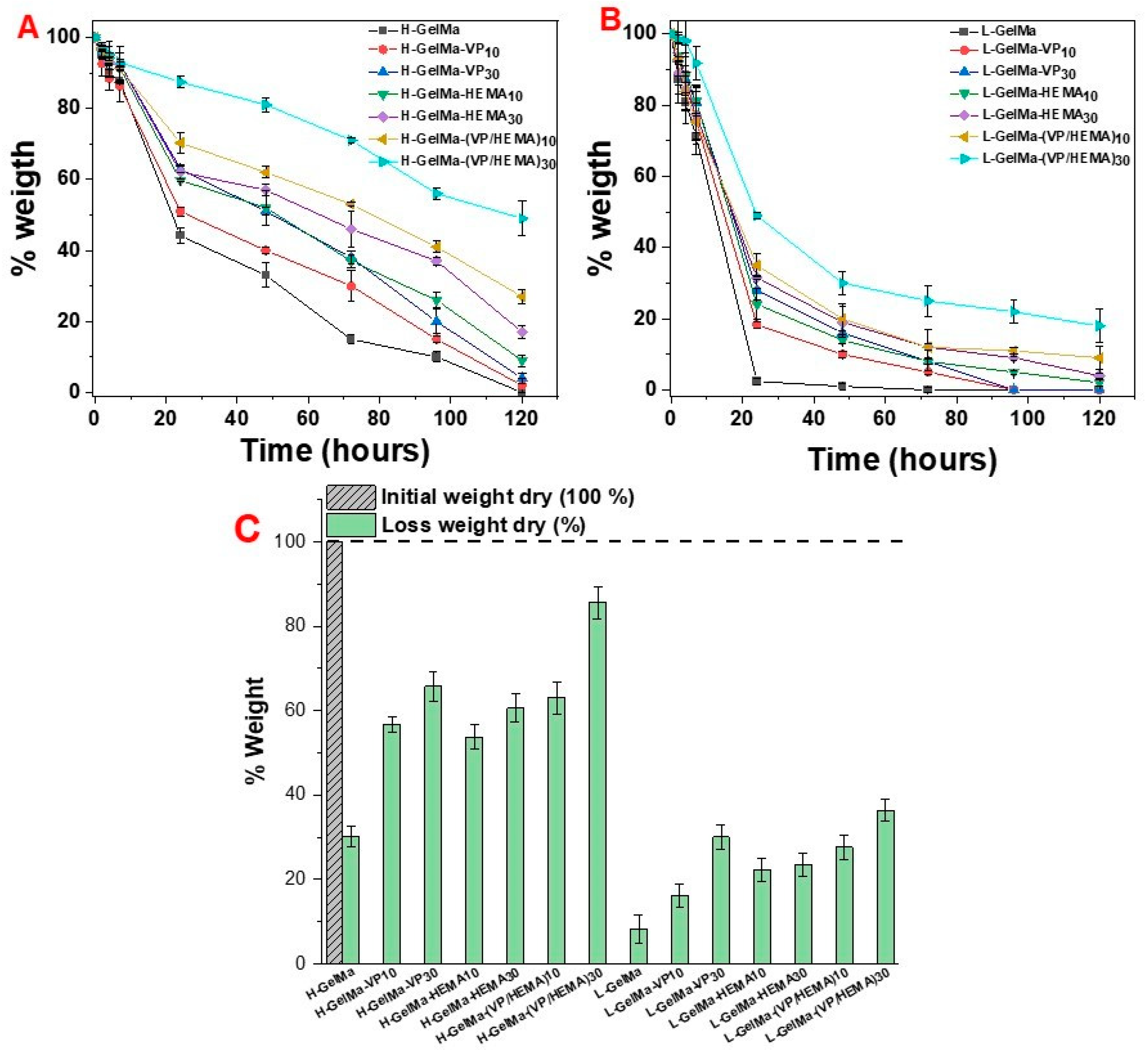
- In vitro enzymatic degradation
- Rheological characterization
| Higher DM | Lower DM | ||||
|---|---|---|---|---|---|
| Acronym | G′ (Pa, at 1 Hz) | G′ (Pa, at 0.1% Strain) | Acronym | G′ (Pa, at 1 Hz) | G′ (Pa, at 0.1% Strain) |
| H-GelMa | 1895 | 1801 | L-GelMa | 813 | 805 |
| H-GelMa-(VP/HEMA)10 | 4796 | 4729 | L-GelMa-(VP/HEMA)10 | 1500 | 1470 |
| H-GelMa-(VP/HEMA)30 | 6770 | 6543 | L-GelMa-(VP/HEMA)30 | 2428 | 2359 |
2.3. Cell Response to Biopolymers
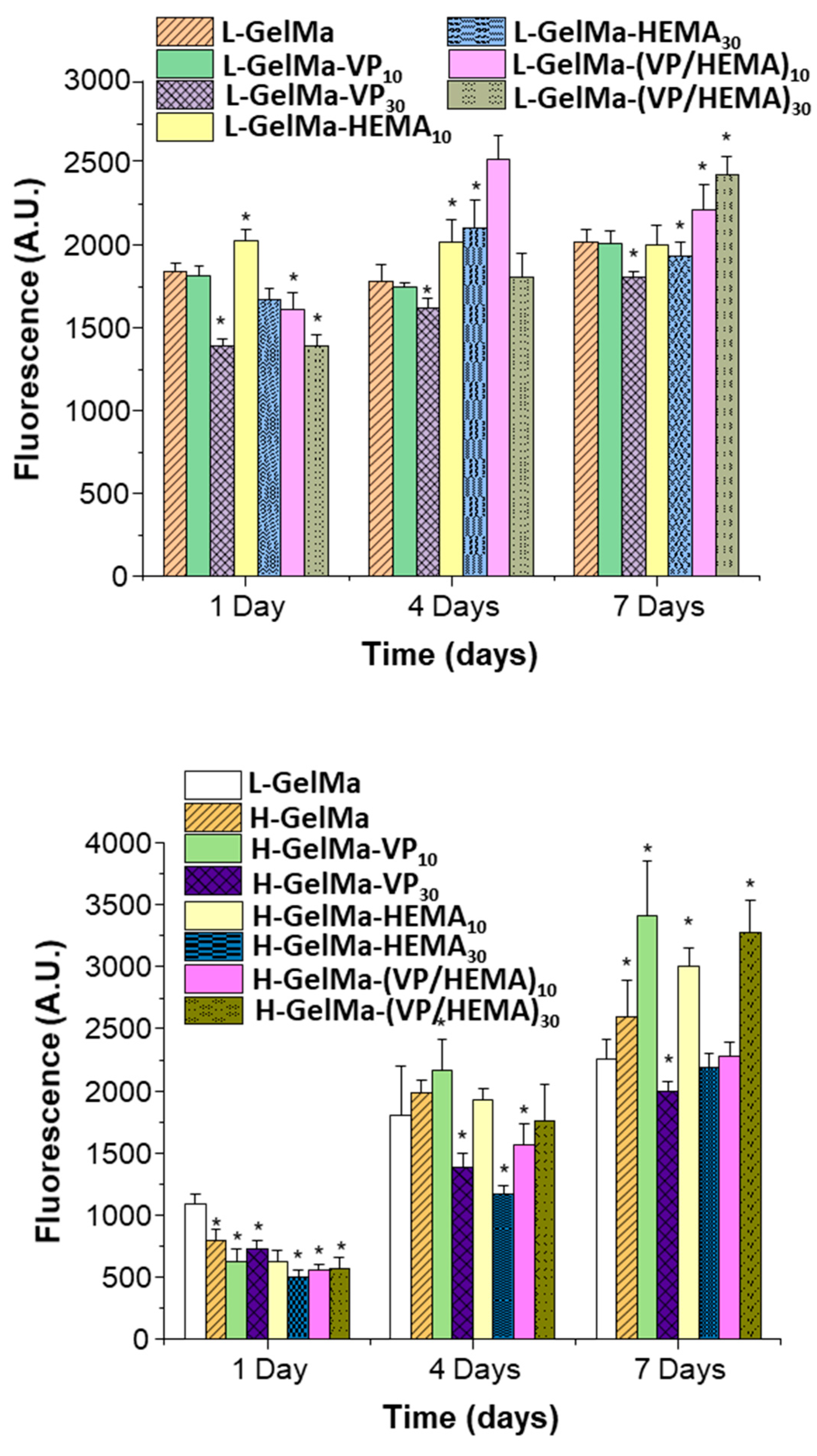
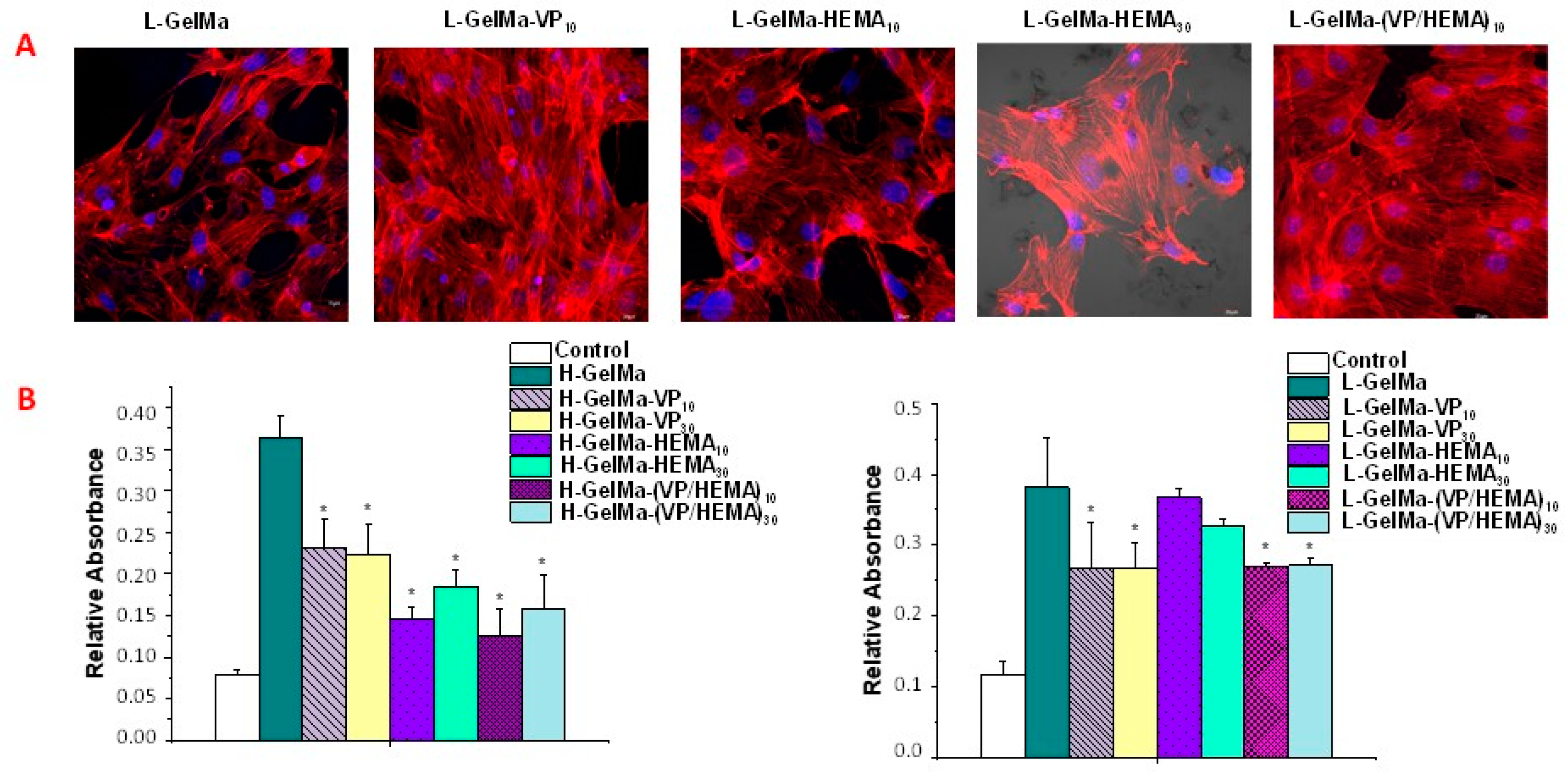
2.3.1. Cell Viability and Proliferation of MC3T3-E1 Cells Exposed to GelMa-Based Biopolymers
2.3.2. Cell Differentiation of MC3T3-E1 Cells Exposed to GelMa Based Copolymers
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis of GelMa with Two Methacryloylation Degrees (DM)
4.3. Degree of Substitution of GelMa Synthesized
4.4. Foamed Biomaterial Preparation: GelMa and GelMa Copolymers
4.5. GelMa and GelMa Based Copolymers Characterization: Instrumentation and Methods
4.6. Swelling Experiments
4.7. In Vitro Enzymatic Degradation
4.8. In Vitro Assays in Pre-Osteoblastic Mammalian Cells
4.9. In Vitro Cytocompatibility Assays in Pre-Osteoblastic Mammalian Cells
4.10. Morphological Studies by Confocal Laser Scanning Microscopy
4.11. In Vitro Mineralization Assay
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Stiff Gelatin Hydrogels Can Be Photo-Chemically Synthesized from Low Viscous Gelatin Solutions Using Molecularly Functionalized Gelatin with a High Degree of Methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Alexa, R.L.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.M.; Ianchis, R. 3D-Printed Gelatin Methacryloyl-Based Scaffolds with Potential Application in Tissue Engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Ghazali, H.S.; Ghazali, Z.S.; Bhattacharyya, A.; Noh, I. Progress in Biomechanical Stimuli on the Cell-Encapsulated Hydrogels for Cartilage Tissue Regeneration. Biomater. Res. 2023, 27, 22. [Google Scholar] [CrossRef]
- Yu, L.; Cavelier, S.; Hannon, B.; Wei, M. Recent Development in Multizonal Scaffolds for Osteochondral Regeneration. Bioact. Mater. 2023, 25, 122–159. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Holguín, J.; López-Hidalgo, A.; Sánchez-Salcedo, S.; Peña, J.; Vallet-Regí, M.; Salinas, A.J. Strontium-Modified Scaffolds Based on Mesoporous Bioactive Glasses/Polyvinyl Alcohol Composites for Bone Regeneration. Materials 2020, 13, 5526. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.; Bu, Z.; Liu, Z.; Lv, F.; Pan, M.; Huang, X.; Cheng, L. Small Extracellular Vesicles Released from Bioglass/Hydrogel Scaffold Promote Vascularized Bone Regeneration by Transferring MiR-23a-3p. Int. J. Nanomed. 2022, 17, 6201–6220. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin Methacryloyl (GelMA)-Based Biomaterials for Bone Regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Kolahdoozan, M.; Rahimi, T.; Taghizadeh, A.; Aghaei, H. Preparation of New Hydrogels by Visible Light Cross-Linking of Dextran Methacrylate and Poly(Ethylene Glycol)-Maleic Acid Copolymer. Int. J. Biol. Macromol. 2023, 227, 1221–1233. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, Printing, and Engineering of Regenerative Biomaterials for Personalized Bone Healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar] [CrossRef]
- Pablos, J.L.; Cicuéndez, M.; Hernández-Rivas, M.; Catalina, F.; Vallet-Regí, M.; Corrales, T. New PCL/PEC Blends: In Vitro Cell Response of Preosteoblasts and Human Mesenchymal Stem Cells. Biology 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Castro, N.J.; Lee, S.J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced Bone Tissue Regeneration Using a 3D Printed Microstructure Incorporated with a Hybrid Nano Hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Gawlitta, D.; Benders, K.E.M.; Toma, S.M.H.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Endochondral Bone Formation in Gelatin Methacrylamide Hydrogel with Embedded Cartilage-Derived Matrix Particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, M.; Cheng, J.; Liu, X.; Liu, Z.; Liu, J.; Tang, P. 3D-Printed GelMA/PEGDA/F127DA Scaffolds for Bone Regeneration. J. Funct. Biomater. 2023, 14, 96. [Google Scholar] [CrossRef]
- Shalchy, F.; Lovell, C.; Bhaskar, A. Hierarchical Porosity in Additively Manufactured Bioengineering Scaffolds: Fabrication & Characterisation. J. Mech. Behav. Biomed. Mater. 2020, 110, 103968. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Piao, Y.; You, H.; Xu, T.; Bei, H.P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical Applications of Gelatin Methacryloyl Hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.H.; Kweon, O.K. Effect of Gelatin on Osteogenic Cell Sheet Formation Using Canine Adipose-Derived Mesenchymal Stem Cells. Cell. Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a Delivery Vehicle for the Controlled Release of Bioactive Molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef]
- Dragusin, D.M.; Van Vlierberghe, S.; Dubruel, P.; Dierick, M.; Van Hoorebeke, L.; Declercq, H.A.; Cornelissen, M.M.; Stancu, I.C. Novel Gelatin-PHEMA Porous Scaffolds for Tissue Engineering Applications. Soft Matter 2012, 8, 9589–9602. [Google Scholar] [CrossRef]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and Characterization of Gelatin-PVP Polymer Composite Scaffold for Potential Application in Bone Tissue Engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Grytsenko, O.; Dulebova, L.; Suberlyak, O.; Skorokhoda, V.; Spišák, E.; Gajdoš, I. Features of Structure and Properties of Phema-Gr-Pvp Block Copolymers, Obtained in the Presence of Fe2+. Materials 2020, 13, 4580. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, J.R.; Chen, L.; Zhu, J.; Hu, Z.M. Preparation and Properties of Morphology Controlled Poly(2-Hydroxyethyl Methacrylate)/Poly(N-Vinyl Pyrrolidone) Double Networks for Biomedical Use. Curr. Appl. Phys. 2010, 10, 766–770. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell Loaded GelMA:HEMA IPN Hydrogels for Corneal Stroma Engineering. J. Mater. Sci. Mater. Med. 2020, 31, 2. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical Tailoring of Gelatin to Adjust Its Chemical and Physical Properties for Functional Bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Shirahama, H.; Cho, N.J.; Tan, L.P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Mokry, J.; Karbanova, J.; Lukas, J.; Paleckova, V.; Dvorankova, B. Biocompatibility of HEMA Copolymers Designed for Treatment of CNS Diseases with Polymer-Encapsulated Cells. Biotechnol. Prog. 2000, 16, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Liao, J.; Tan, Y.; Ouyang, K.; Ning, C.; Ni, G.; Tan, G. Cell-Laden Photocrosslinked GelMA-DexMA Copolymer Hydrogels with Tunable Mechanical Properties for Tissue Engineering. J. Mater. Sci. Mater. Med. 2014, 25, 2173–2183. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mu, C.; Lin, W. Novel Hemocompatible Nanocomposite Hydrogels Crosslinked with Methacrylated Gelatin. RSC Adv. 2016, 6, 43663–43671. [Google Scholar] [CrossRef]
- Şarkaya, K.; Allı, A. Synthesis and Characterization of Cryogels of p(HEMA-N-Vinylformamide) and p(HEMA-N-Vinylpyrrolidone) for Chemical Release Behaviour. J. Porous Mater. 2021, 28, 853–865. [Google Scholar] [CrossRef]
- Erkoc, P.; Seker, F.; Bagci-Onder, T.; Kizilel, S. Gelatin Methacryloyl Hydrogels in the Absence of a Crosslinker as 3D Glioblastoma Multiforme (GBM)-Mimetic Microenvironment. Macromol. Biosci. 2018, 18, 1700369. [Google Scholar] [CrossRef]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Maciel Filho, R. PHEMA Hydrogels: Synthesis, Kinetics and in Vitro Tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-Tricalcium Phosphate/Agarose Macroporous Scaffolds for Bone Tissue Engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Wessely, I.D.; Matt, Y.; An, Q.; Bräse, S.; Tsotsalas, M. Dynamic Porous Organic Polymers with Tuneable Crosslinking Degree and Porosity. RSC Adv. 2021, 11, 27714–27719. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P.; Horowitz, D.M.; Peoples, O.P. PHA Applications: Addressing the Price Performance Issue I. Tissue Engineering. Int. J. Biol. Macromol. 1999, 25, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic Principle and Good Practices of Rheology for Polymers for Teachers and Beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A Protocol for Rheological Characterization of Hydrogels for Tissue Engineering Strategies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Bandow, K.; Suzuki, H.; Chiba, N.; Kakimoto, K.; Ohnishi, T.; Kawamoto, S.I.; Nagaoka, E.; Matsuguchi, T. Osteoblast Differentiation Is Functionally Associated with Decreased AMP Kinase Activity. J. Cell. Physiol. 2009, 221, 740–749. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Mimicking Corneal Stroma Using Keratocyte-Loaded Photopolymerizable Methacrylated Gelatin Hydrogels. J. Tissue Eng. Regen. Med. 2018, 12, e1899–e1910. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct Proliferative and Differentiated Stages of Murine MC3T3-E1 Cells in Culture: An in Vitro Model of Osteoblast Development. J. Bone Miner. Res. 2009, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]




| Foamed Biomaterials | |||
|---|---|---|---|
| Higher DM | Lower DM | ||
| Acronym | Composition | Acronym | Composition |
| H-GelMa | 100/0/0/1.5 | L-GelMa | 100/0/0/1.5 |
| H-GelMa-VP10 | 90/10/0/1.5 | L-GelMa-VP10 | 90/10/0/1.5 |
| H-GelMa-VP30 | 70/30/0/1.5 | L-GelMa-VP30 | 70/30/0/1.5 |
| H-GelMa-HEMA10 | 90/10/0/1.5 | L-GelMa-HEMA10 | 90/10/0/1.5 |
| H-GelMa-HEMA30 | 70/30/0/1.5 | L-GelMa-HEMA30 | 70/30/0/1.5 |
| H-GelMa-(VP/HEMA)10 | 90/5/5/1.5 | L-GelMa-(VP/HEMA)10 | 90/5/5/1.5 |
| H-GelMa-(VP/HEMA)30 | 70/15/15/1.5 | L-GelMa-(VP/HEMA)30 | 70/15/15/1.5 |
| T10 (°C) | Tmax (1) (°C) | Tmax (2) (°C) | |
| H-GelMa | 190 | 318 | --- |
| H-GelMa-VP10 | 244 | 325 | 427 |
| H-GelMa-VP30 | 255 | 328 | 432 |
| H-GelMa-HEMA10 | 252 | 325 | 411 |
| H-GelMa-HEMA30 | 260 | 325 | 415 |
| H-GelMa-(VP/HEMA)10 | 253 | 323 | 420 |
| H-GelMa-(VP/HEMA)30 | 261 | 325 | 423 |
| T10 (°C) | Tmax (1) (°C) | Tmax (2) (°C) | |
| L-GelMa | 200 | 319 | --- |
| L-GelMa-VP10 | 248 | 324 | 430 |
| L-GelMa-VP30 | 259 | 325 | 431 |
| L-GelMa-HEMA10 | 249 | 324 | 413 |
| L-GelMa-HEMA30 | 260 | 325 | 414 |
| L-GelMa-(VP/HEMA)10 | 245 | 325 | 420 |
| L-GelMa-(VP/HEMA)30 | 260 | 325 | 423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pablos, J.L.; Jiménez-Holguín, J.; Salcedo, S.S.; Salinas, A.J.; Corrales, T.; Vallet-Regí, M. New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering. Gels 2023, 9, 403. https://doi.org/10.3390/gels9050403
Pablos JL, Jiménez-Holguín J, Salcedo SS, Salinas AJ, Corrales T, Vallet-Regí M. New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering. Gels. 2023; 9(5):403. https://doi.org/10.3390/gels9050403
Chicago/Turabian StylePablos, Jesús L., Javier Jiménez-Holguín, Sandra Sánchez Salcedo, Antonio J. Salinas, Teresa Corrales, and María Vallet-Regí. 2023. "New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering" Gels 9, no. 5: 403. https://doi.org/10.3390/gels9050403