Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range
Abstract
:1. Introduction
2. Results and Discussion
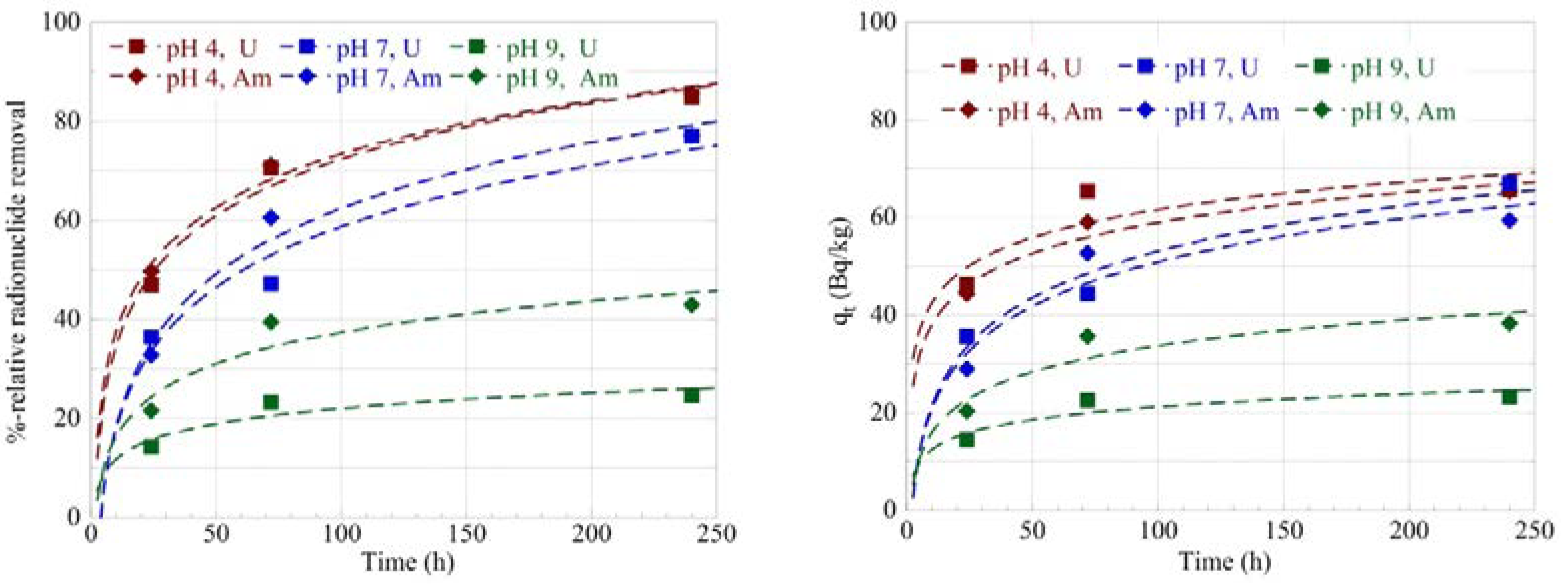
2.1. Effect of Contact Time
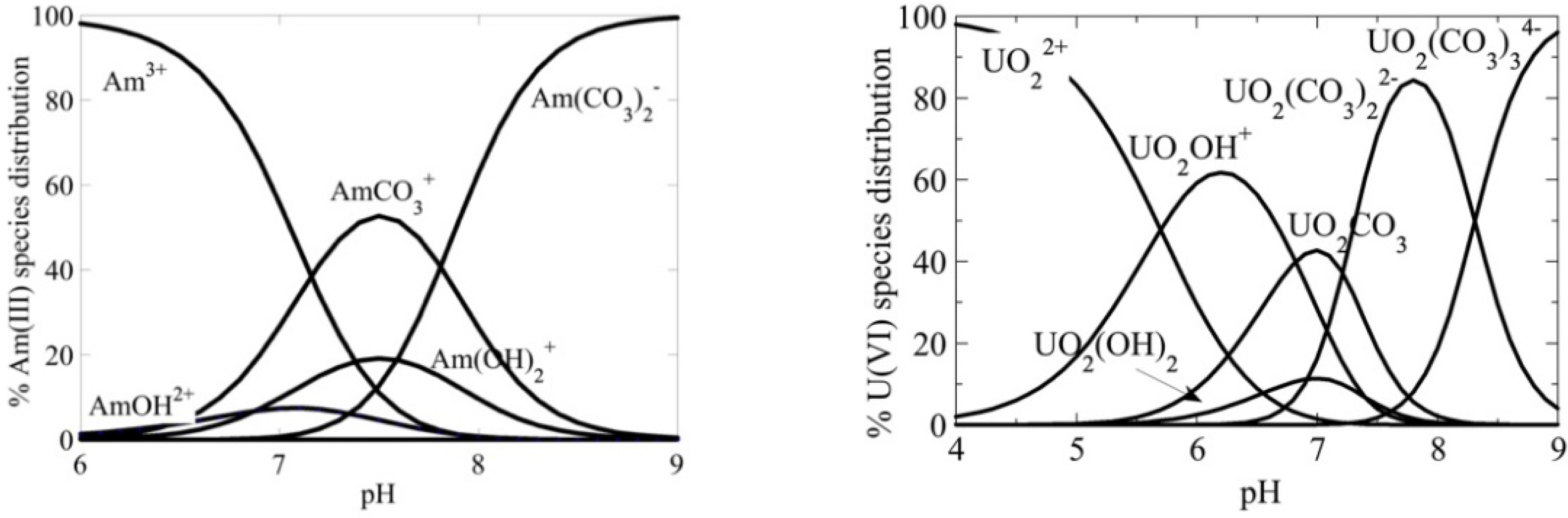
2.2. Constants for the Distribution of Am and U between Aqueous and Aerogel Phase
3. Conclusions
4. Materials and Methods
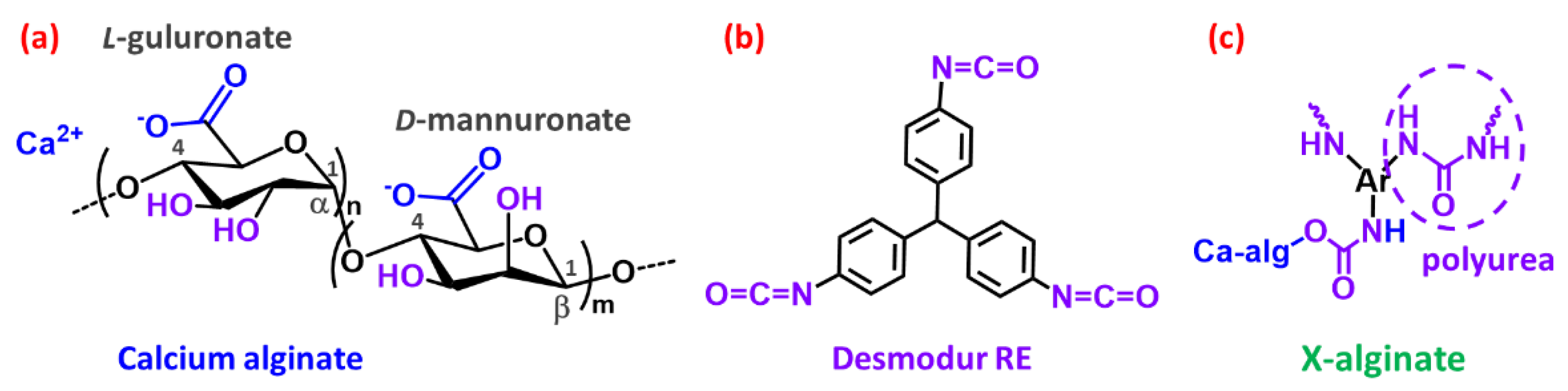
4.1. Preparation and Characterization of X-Alginate Aerogels
4.2. Adsorption Experiments
4.3. Removal of Radionuclides from Environmental Waters
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Dinh Chau, N.; Dulinski, M.; Jodlowski, P.; Nowak, J.; Rozanski, K.; Sleziak, M.; Wachniew, P. Natural Radioactivity in Groundwater–a Review. Isot. Environ. Health Stud. 2011, 47, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click Synthesis of Monolithic Silicon Carbide Aerogels from Polyacrylonitrile-Coated 3D Silica Networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A Reconsideration on the Definition of the Term Aerogel Based on Current Drying Trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly Enhanced Adsorption Performance to Uranium(VI) by Facile Synthesized Hydroxyapatite Aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Design of Hydroxyapatite Aerogel with Excellent Adsorption Performance to Uranium. J. Environ. Chem. Eng. 2021, 9, 106364. [Google Scholar] [CrossRef]
- Zhao, M.; Tesfay Reda, A.; Zhang, D. Reduced Graphene Oxide/ZIF-67 Aerogel Composite Material for Uranium Adsorption in Aqueous Solutions. ACS Omega 2020, 5, 8012–8022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; He, H.; Liu, Z.; Lai, Z.; Wang, Y. A Facile Method for Preparing Three-Dimensional Graphene Nanoribbons Aerogel for Uranium(VI) and Thorium(IV) Adsorption. J. Radioanal. Nucl. Chem. 2021, 328, 289–298. [Google Scholar] [CrossRef]
- Georgiou, E.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Efficient Removal of Polyvalent Metal Ions (Eu(III) and Th(IV)) from Aqueous Solutions by Polyurea-Crosslinked Alginate Aerogels. Gels 2022, 8, 478. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Hu, X.; Wu, P.; Lan, T.; Li, Y.; Tu, H.; Liu, Y.; Yuan, D.; Wu, Z.; et al. Synthesis and Characterization of Poly(TRIM/VPA) Functionalized Graphene Oxide Nanoribbons Aerogel for Highly Efficient Capture of Thorium(IV) from Aqueous Solutions. Appl. Surf. Sci. 2021, 536, 147829. [Google Scholar] [CrossRef]
- Bai, R.; Yang, F.; Meng, L.; Zhao, Z.; Guo, W.; Cai, C.; Zhang, Y. Polyethylenimine Functionalized and Scaffolded Graphene Aerogel and the Application in the Highly Selective Separation of Thorium from Rare Earth. Mater. Des. 2021, 197, 109195. [Google Scholar] [CrossRef]
- Riley, B.J.; Chun, J.; Um, W.; Lepry, W.C.; Matyas, J.; Olszta, M.J.; Li, X.; Polychronopoulou, K.; Kanatzidis, M.G. Chalcogen-Based Aerogels As Sorbents for Radionuclide Remediation. Environ. Sci. Technol. 2013, 47, 7540–7547. [Google Scholar] [CrossRef]
- Lee, I.; Kim, S.-H.; Rethinasabapathy, M.; Haldorai, Y.; Lee, G.-W.; Choe, S.R.; Jang, S.-C.; Kang, S.-M.; Han, Y.-K.; Roh, C.; et al. Porous 3D Prussian Blue/Cellulose Aerogel as a Decorporation Agent for Removal of Ingested Cesium from the Gastrointestinal Tract. Sci. Rep. 2018, 8, 4540. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Li, Y.; Yu, Y.; Duan, T.; Zhou, D.; Wang, L.; Zhou, J.; Kuang, M. Environment-Friendly Bio-Materials Based on Cotton-Carbon Aerogel for Strontium Removal from Aqueous Solution. J. Radioanal. Nucl. Chem. 2018, 316, 553–560. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. A Basic Toxicity Classification of Radionuclides; International Atomic Energy Agency: Vienna, Austria, 1963; Available online: https://www.iaea.org/publications/1110/a-basic-toxicity-classification-of-radionuclides (accessed on 6 March 2023).
- Choppin, G.R.; Jensen, M.P. Actinides in Solution: Complexation and Kinetics. In The Chemistry of the Actinide and Transactinide Elements, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011; p. 2524. [Google Scholar]
- Denecke, M.A.; Bryan, N.; Kalmykov, S.; Morris, K.; Quinto, F. Sources and Behaviour of Actinide Elements in the Environment. In Experimental and Theoretical Approaches to Actinide Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 378–444. ISBN 978-1-119-11555-7. [Google Scholar]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Chatzichristidi, M.; Raptopoulos, G.; Gurikov, P. Mechanically Strong Polyurea/Polyurethane-Cross-Linked Alginate Aerogels. ACS Appl. Polym. Mater. 2020, 2, 1974–1988. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Raptopoulos, G.; Gurikov, P. Polyurea-Crosslinked Biopolymer Aerogel Beads. RSC Adv. 2020, 10, 40843. [Google Scholar] [CrossRef]
- Raptopoulos, G.; Papastergiou, M.; Chriti, D.; Effraimopoulou, E.; Čendak, T.; Samartzis, N.; Mali, G.; Ioannides, T.; Gurikov, P.; Smirnova, I.; et al. Metal-Doped Carbons from Polyurea-Crosslinked Alginate Aerogel Beads. Mater. Adv. 2021, 2, 2684–2699. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Raptopoulos, G.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Fundamental Skeletal Nanostructure of Nanoporous Polymer-Cross-Linked Alginate Aerogels and Its Relevance to Environmental Remediation. ACS Appl. Nano Mater. 2021, 4, 10575–10583. [Google Scholar] [CrossRef]
- Fricke, M.; Paraskevopoulou, P.; Gurikov, P.; Chriti, D.; Papastergiou, M.; Raptopoulos, G.; Athamneh, T.; Smirnova, I.; Movahed, S.; Weinrich, D.; et al. Polyurea/Polyurethane-Crosslinked Alginate Aerogels. EP3848409A1, 14 July 2021. [Google Scholar]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically Strong Aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Mandal, C.; Donthula, S.; Far, H.M.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Transparent, Mechanically Strong, Thermally Insulating Cross-Linked Silica Aerogels for Energy-Efficient Windows. J. Sol-Gel Sci. Technol. 2019, 92, 84–100. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene Aerogels Grafted with PMMA: I. Molecular and Interparticle Crosslinking. Soft Matter 2013, 9, 1516–1530. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene Aerogels Grafted with PMMA: II. Nanoscopic Characterization and Origin of Macroscopic Deformation. Soft Matter 2013, 9, 1531–1539. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Macroporous Electrically Conducting Carbon Networks by Pyrolysis of Isocyanate-Cross-Linked Resorcinol-Formaldehyde Aerogels. Chem. Mater. 2008, 20, 6985–6997. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Raptopoulos, G.; Leontaridou, F.; Papastergiou, M.; Sakellari, A.; Karavoltsos, S. Evaluation of Polyurea-Crosslinked Alginate Aerogels for Seawater Decontamination. Gels 2021, 7, 27. [Google Scholar] [CrossRef]
- Ghimire, S.; Sala, M.R.; Chandrasekaran, S.; Raptopoulos, G.; Worsley, M.; Paraskevopoulou, P.; Leventis, N.; Sabri, F. Noninvasive Detection, Tracking, and Characterization of Aerogel Implants Using Diagnostic Ultrasound. Polymers 2022, 14, 722. [Google Scholar] [CrossRef]
- Sabri, F.; Sebelik, M.E.; Meacham, R.; Boughter, J.D., Jr.; Challis, M.J.; Leventis, N. In Vivo Ultrasonic Detection of Polyurea Crosslinked Silica Aerogel Implants. PLoS ONE 2013, 8, e66348. [Google Scholar] [CrossRef] [Green Version]
- Sabri, F.; Cole, J.A.; Scarbrough, M.C.; Leventis, N. Investigation of Polyurea-Crosslinked Silica Aerogels as a Neuronal Scaffold: A Pilot Study. PLoS ONE 2012, 7, e33242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchero, P.; Painter, S.; Ebrahimi, H.; Koskinen, L.; Molinero, J.; Selroos, J.-O. Modelling Radionuclide Transport in Fractured Media with a Dynamic Update of Kd Values. Comput. Geosci. 2016, 86, 55–63. [Google Scholar] [CrossRef]
- Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Microplastics as Radionuclide (U-232) Carriers. J. Mol. Liq. 2022, 351, 118641. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Neptunium Interaction with Microplastics in Aqueous Solutions. J. Mol. Liq. 2022, 356, 119056. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Theocharis, C.R. Uranium Isotope (U-232) Removal from Waters by Biochar Fibers: An Adsorption Study in the Sub-Picomolar Concentration Range. Molecules 2022, 27, 6765. [Google Scholar] [CrossRef]
- Mühr-Ebert, E.L.; Wagner, F.; Walther, C. Speciation of Uranium: Compilation of a Thermodynamic Database and Its Experimental Evaluation Using Different Analytical Techniques. Appl Geochem 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Konstantinou, M.; Pashalidis, I. Speciation and Spectrophotometric Determination of Uranium in Seawater. Mediterr. Mar. Sci. 2004, 5, 55–60. [Google Scholar] [CrossRef]
- Stadler, S.; Kim, J.I. Hydrolysis Reactions of Am (III) and Am(V). Radiochim. Acta 1988, 44–45, 39–44. [Google Scholar] [CrossRef]
- Meinrath, G.; Kim, J.I. The Carbonate Complexation of the Am(III) Ion. Radiochim. Acta 1991, 52–53, 29–34. [Google Scholar] [CrossRef]
- Runde, W.; Meinrath, G.; Kim, J.I. A Study of Solid-Liquid Phase Equilibria of Trivalent Lanthanide and Actinide Ions in Carbonate Systems. Radiochim. Acta 1992, 58–59, 93–100. [Google Scholar] [CrossRef]
- Choppin, G.R. Comparison of the Solution Chemistry of the Actinides and Lanthanides. J. Less Common Met. 1983, 93, 323–330. [Google Scholar] [CrossRef]
- Ramírez-Guinart, O.; Kaplan, D.; Rigol, A.; Vidal, M. Deriving Probabilistic Soil Distribution Coefficients (Kd). Part 3: Reducing Variability of Americium Kd Best Estimates Using Soil Properties and Chemical and Geological Material Analogues. J. Environ. Radioact. 2020, 223–224, 106378. [Google Scholar] [CrossRef]
- Sheppard, S.C.; Sheppard, M.I.; Tait, J.C.; Sanipelli, B.L. Revision and Meta-Analysis of Selected Biosphere Parameter Values for Chlorine, Iodine, Neptunium, Radium, Radon and Uranium. J. Environ. Radioact. 2006, 89, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, M.; Pashalidis, I. A Comparative Study on the Sorption of Tri- and Hexavalent Actinides on Sea Sediments. J. Radioanal. Nucl. Chem. 2017, 312, 181–185. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Single-Use Surgical Face Masks as Radionuclide (U-232 and Ra-226) Carriers. J. Mol. Liq. 2021, 342, 117578. [Google Scholar] [CrossRef]
- American Public Health Association; Eaton, A.D.; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005; ISBN 978-0-87553-047-5. [Google Scholar]


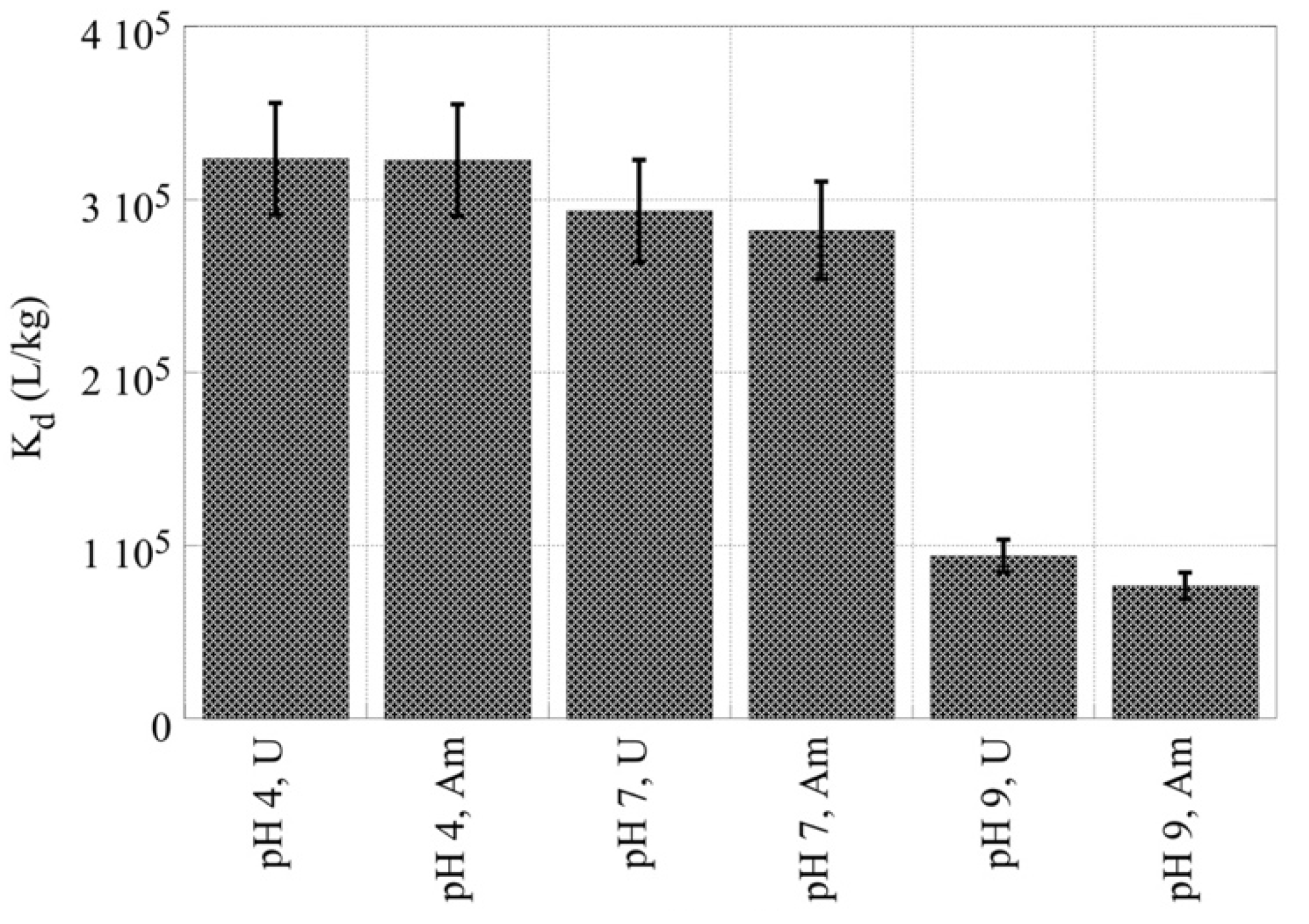

| Kd (L/kg) U-232 | Kd (L/kg) Am-241 | |
|---|---|---|
| Laboratory solutions | ||
| pH 4 | 3.2 × 105 | 3.2 × 105 |
| pH 7 | 2.9 × 105 | 2.8 × 105 |
| pH 9 | 9.4 × 104 | 7.7 × 104 |
| Environmental waters | ||
| Seawater (SW) | 9.6 × 104 | 8.6 × 104 |
| Ground water (GW) | 1.1 × 105 | 1.5 × 105 |
| Wastewater (WW) | 1.2 × 105 | 9.9 × 104 |
| Parameter | WW | GW | SW |
|---|---|---|---|
| pH | 8.1 | 7.8 | 8.3 |
| K+ (mg L−1) | 29 | <3 | 395 |
| Na+ (mg L−1) | - | 40 | 10,680 |
| Ca2+ (mg L−1) | 87 | 38 | 410 |
| Mg2+ (mg L−1) | 55 | 70 | 1280 |
| Fe3+ (mg L−1) | - | <35 | 0.003 |
| Cu2+ (mg L−1) | - | <50 | 0.09 |
| Cl− (mg L−1) | 298 | 54 | 19,200 |
| HCO3− (mg L−1) | - | 370 | 140 |
| SO42– (mg L−1) | 111 | 95 | 2680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, I.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range. Gels 2023, 9, 211. https://doi.org/10.3390/gels9030211
Ioannidis I, Pashalidis I, Raptopoulos G, Paraskevopoulou P. Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range. Gels. 2023; 9(3):211. https://doi.org/10.3390/gels9030211
Chicago/Turabian StyleIoannidis, Ioannis, Ioannis Pashalidis, Grigorios Raptopoulos, and Patrina Paraskevopoulou. 2023. "Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range" Gels 9, no. 3: 211. https://doi.org/10.3390/gels9030211