Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties
Abstract
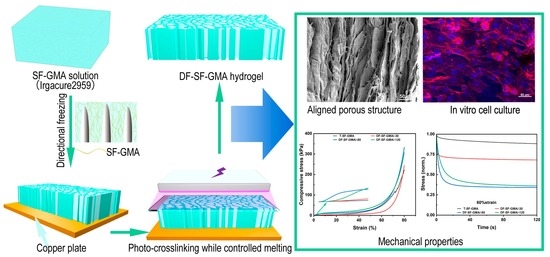
:1. Introduction
2. Results and Discussion
2.1. SF-GMA Characterization and Hydrogel Secondary Structure
2.2. The Porous Architecture
2.3. Mechanical Properties of SF-GMA
2.4. Viscoelastic Properties of SF-GMA Hydrogels
2.5. Cell Biocompatibility
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis of SF-GMA Macromer and 1H NMR
4.3. Preparation of SF-GMA Hydrogel
4.4. Structure Characterization
4.5. FTIR Analysis
4.6. Mechanical Characteristics
4.7. Swelling Ratio Measurement
4.8. Biocompatibility
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Jing, Z.; Naoki, K.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, Y.; Liu, S.; Du, Y.; Zhang, S.; Wang, J. Freeze-casting osteochondral scaffolds: The presence of a nutrient-permeable film between the bone and cartilage defect reduces cartilage regeneration. Acta Biomater. 2022, 154, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels with Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [Green Version]
- Milazzo, L.; Vulcano, F.; Macioce, G.; Marziali, G.; Iosi, F.; Bertuccini, L.; Falchi, M.; Rech, F.; Giampaolo, A.; Pecci, R.; et al. Silk Fibroin Scaffolds as Biomaterials for 3D Mesenchymal Stromal Cells Cultures. Appl. Sci. 2021, 11, 11345. [Google Scholar] [CrossRef]
- Ma, Y.; Duan, L.; Sun, J.; Gou, S.; Chen, F.; Liang, Y.; Dai, F.; Xiao, B. Oral nanotherapeutics based on Antheraea pernyi silk fibroin for synergistic treatment of ulcerative colitis. Biomaterials 2022, 282, 121410. [Google Scholar] [CrossRef]
- Long, D.; Xiao, B.; Merlin, D. Genetically modified silk fibroin nanoparticles for drug delivery: Preparation strategies and application prospects. Nanomedicine 2020, 15, 1739–1742. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Aoki, A.; Nakazawa, Y.; Knight, D.P.; Asakura, T. Structural Analysis of the Synthetic Peptide (Ala-Gly-Ser-Gly-Ala-Gly)5, a Model for the Crystalline Domain of Bombyx mori Silk Fibroin, Studied with 13C CP/MAS NMR, REDOR, and Statistical Mechanical Calculations. Macromolecules 2010, 43, 9434–9440. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically crosslinked silk fibroin/hyaluronic acid scaffolds. Carbohydr. Polym. 2020, 239, 116232. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ning, H.; Liu, S.; Lu, Q.; Fan, Z.; Lu, H.; Lu, G.; Kaplan, D.L. Silk Biomaterials with Vascularization Capacity. Adv. Funct. Mater. 2016, 26, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, X.; Yu, F.; Ma, L.; Pan, X.; Luo, G.; Lin, S.; Mo, X.; He, C.; Wang, H. Hyaluronic acid/EDC/NHS-crosslinked green electrospun silk fibroin nanofibrous scaffolds for tissue engineering. RSC Adv. 2016, 6, 99720–99728. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Ji, Z.; Zhang, Y.; Zhang, P.; He, Y.; Shen, Y. In vitro biocompatibility study of EDC/NHS cross-linked silk fibroin scaffold with olfactory ensheathing cells. J. Biomater. Sci. Polym. Ed. 2022, 34, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Dadashzadeh, A.; Moghassemi, S.; Zahedi, P.; Amorim, C.A.; Shavandi, A. Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties. Gels 2021, 7, 138. [Google Scholar] [CrossRef]
- Shen, H.; Wang, P.; Han, X.; Ma, M.; Shang, Y.; Ju, Y.; Shen, S.; Yin, F.; Wang, Q. GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair. Gels 2022, 8, 56. [Google Scholar] [CrossRef]
- Cui, X.; Soliman, B.G.; Alcala-Orozco, C.R.; Li, J.; Vis, M.A.M.; Santos, M.; Wise, S.G.; Levato, R.; Malda, J.; Woodfield, T.B.F.; et al. Rapid Photocrosslinking of Silk Hydrogels with High Cell Density and Enhanced Shape Fidelity. Adv. Healthc. Mater. 2020, 9, 1901667. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zheng, J.; Liu, L.; Zhang, Q. Three-Dimensional Printing Self-Healing Dynamic/Photocrosslinking Gelatin-Hyaluronic Acid Double-Network Hydrogel for Tissue Engineering. ACS Omega 2022, 7, 12076–12088. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous alginate scaffolds assembled using vaterite CaCO3 crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Kawazoe, N.; Chen, G. Influence of microporous gelatin hydrogels on chondrocyte functions. J. Mater. Chem. B 2017, 5, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lee, K.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Interconnected collagen porous scaffolds prepared with sacrificial PLGA sponge templates for cartilage tissue engineering. J. Mater. Chem. B 2021, 9, 8491–8500. [Google Scholar] [CrossRef]
- Francis, N.L.; Hunger, P.M.; Donius, A.E.; Riblett, B.W.; Zavaliangos, A.; Wegst, U.G.K.; Wheatley, M.A. An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3493–3503. [Google Scholar] [CrossRef]
- Chen, S.; Nakamoto, T.; Kawazoe, N.; Chen, G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials 2015, 73, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cooper, A.I. Aligned Porous Structures by Directional Freezing. Adv. Mater. 2007, 19, 1529–1533. [Google Scholar] [CrossRef]
- Yamamoto, T.; Randriantsilefisoa, R.; Sprecher, C.M.; D’Este, M. Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules 2022, 12, 1809. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Chen, S.; Kawazoe, N.; Chen, G. Biomimetic Assembly of Vascular Endothelial Cells and Muscle Cells in Microgrooved Collagen Porous Scaffolds. Tissue Eng. Part C Methods 2017, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Chen, Y.; Delattre, B.; Tomsia, A.P.; Ritchie, R.O. Bioinspired large-scale aligned porous materials assembled with dual temperature gradients. Sci. Adv. 2015, 1, e1500849. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wu, M.; Gao, W.; Bai, H. A sustainable single-component “Silk nacre”. Sci. Adv. 2022, 8, eabo0946. [Google Scholar] [CrossRef]
- Autissier, A.; Visage, C.L.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xiao, L.; Ding, Z.; Lu, Q.; Kaplan, D.L. Engineered Tough Silk Hydrogels through Assembling β-Sheet Rich Nanofibers Based on a Solvent Replacement Strategy. ACS Nano 2022, 16, 10209–10218. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Cortes, G.; Kumar, U.; Sakthivel, T.S.; Niemiec, S.M.; Louiselle, A.E.; Azeltine-Bannerman, M.; Zgheib, C.; Liechty, K.W.; Seal, S. Silk fibroin nanofibrous mats for visible sensing of oxidative stress in cutaneous wounds. Biomater. Sci. 2020, 8, 5900–5910. [Google Scholar] [CrossRef]
- Lyu, H.; Li, J.; Yuan, Z.; Liu, H.; Sun, Z.; Jiang, R.; Yu, X.; Hu, Y.; Pei, Y.; Ding, J.; et al. Supertough and highly stretchable silk protein-based films with controlled biodegradability. Acta Biomater. 2022, 153, 149–158. [Google Scholar] [CrossRef]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; van Enckevort, W.J.P.; van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; van Kuppevelt, T.H. Versatile Wedge-Based System for the Construction of Unidirectional Collagen Scaffolds by Directional Freezing: Practical and Theoretical Considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [Green Version]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Zhang, Y. Stiffness of the aligned fibers affects structural and functional integrity of the oriented endothelial cells. Acta Biomater. 2020, 108, 237–249. [Google Scholar] [CrossRef]
- Ghaderinejad, P.; Najmoddin, N.; Bagher, Z.; Saeed, M.; Karimi, S.; Simorgh, S.; Pezeshki-Modaress, M. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering. Chem. Eng. J. 2021, 420, 130465. [Google Scholar] [CrossRef]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Li, B.; Zhang, Y. Stiffness of Aligned Fibers Regulates the Phenotypic Expression of Vascular Smooth Muscle Cells. ACS Appl. Mater. Interfaces 2019, 11, 6867–6880. [Google Scholar] [CrossRef]
- Luo, Z.; Tang, G.; Ravanbakhsh, H.; Li, W.; Wang, M.; Kuang, X.; Garciamendez-Mijares, C.E.; Lian, L.; Yi, S.; Liao, J. Vertical extrusion cryo (bio) printing for anisotropic tissue manufacturing. Adv. Mater. 2022, 34, 2108931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Ice Templating and Freeze-Drying for Porous Materials and Their Applications; John Wiley & Sons: New York, NY, USA, 2018. [Google Scholar]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Sun, Q.; Li, Q.; Li, X. Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties. Gels 2023, 9, 181. https://doi.org/10.3390/gels9030181
Jiang Z, Sun Q, Li Q, Li X. Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties. Gels. 2023; 9(3):181. https://doi.org/10.3390/gels9030181
Chicago/Turabian StyleJiang, Zewu, Qingqing Sun, Qian Li, and Xiaomeng Li. 2023. "Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties" Gels 9, no. 3: 181. https://doi.org/10.3390/gels9030181