Spatial Control over Catalyst Positioning for Increased Micromotor Efficiency
Abstract
:1. Introduction
2. Results and Discussion
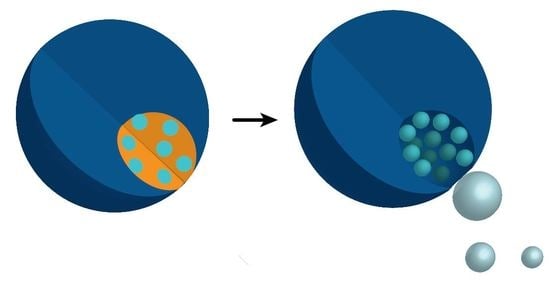
2.1. Experimental Design
2.2. Catalyst Distribution
2.3. Motion Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. PDMS Microfluidic Device
4.3. Micromotor Fabrication
4.4. Activity Assay
4.5. Autonomous Movement Experiments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrede, P.; Medina-Sánchez, M.; Fomin, V.M.; Schmidt, O.G. Switching Propulsion Mechanisms of Tubular Catalytic Micromotors. Small 2021, 17, 2006449. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Pumera, M. Crucial Role of Surfactants in Bubble-Propelled Microengines. J. Phys. Chem. C 2014, 118, 5268–5274. [Google Scholar] [CrossRef]
- Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines 2017, 8, 267. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, F.; Qiao, R.; Zhao, Y. Marangoni Flow Induced Collective Motion of Catalytic Micromotors. J. Phys. Chem. C 2015, 119, 28361–28367. [Google Scholar] [CrossRef]
- Mao, Z.; Shimamoto, G.; Maeda, S. Conical frustum gel driven by the Marangoni effect for a motor without a stator. Colloids Surf. A 2021, 608, 125561. [Google Scholar] [CrossRef]
- Keller, S.; Hu, G.X.; Gherghina-Tudor, M.I.; Teora, S.P.; Wilson, D.A. A Microfluidic Tool for Fine-Tuning Motion of Soft Micromotors. Adv. Funct. Mater. 2019, 29, 1904889. [Google Scholar] [CrossRef]
- Wang, S.; Wu, N. Selecting the Swimming Mechanisms of Colloidal Particles: Bubble Propulsion versus Self-Diffusiophoresis. Langmuir 2014, 30, 3477–3486. [Google Scholar] [CrossRef]
- Zhao, G.; Pumera, M. Geometric asymmetry driven Janus micromotors. Nanoscale 2014, 6, 11177–11180. [Google Scholar] [CrossRef]
- Wang, L.; Borrelli, M.; Simmchen, J. Self-Asymmetric Yolk–Shell Photocatalytic ZnO Micromotors. ChemPhotoChem 2021, 5, 933–939. [Google Scholar] [CrossRef]
- Su, M.; Liu, M.; Liu, L.; Sun, Y.; Li, M.; Wang, D.; Zhang, H.; Dong, B. Shape-Controlled Fabrication of the Polymer-Based Micromotor Based on the Polydimethylsiloxane Template. Langmuir 2015, 31, 11914–11920. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y. Autonomous motion of immobilized enzyme on Janus particles significantly facilitates enzymatic reactions. Biochem. Eng. J. 2019, 149, 107242. [Google Scholar] [CrossRef]
- Teora, S.P.; van der Knaap, K.H.; Keller, S.; Rijpkema, S.J.; Wilson, D.A. Reversible speed control of one-stimulus-double-response, temperature-sensitive asymmetric hydrogel micromotors. Chem. Commun. 2022, 58, 10333–10336. [Google Scholar] [CrossRef]
- Ji, Y.; Lin, X.; Wang, D.; Zhou, C.; Wu, Y.; He, Q. Continuously Variable Regulation of the Speed of Bubble-Propelled Janus Microcapsule Motors Based on Salt-Responsive Polyelectrolyte Brushes. Chem.-Asian J. 2019, 14, 2450–2455. [Google Scholar] [CrossRef]
- Sun, J.; Mathesh, M.; Li, W.; Wilson, D.A. Enzyme-Powered Nanomotors with Controlled Size for Biomedical Applications. ACS Nano 2019, 13, 10191–10200. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, H.; Yasa, I.C.; Sitti, M. 3D Chemical Patterning of Micromaterials for Encoded Functionality. Adv. Mater. 2017, 29, 1605072. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Mundaca-Uribe, R.; Wei, X.; Ramírez-Herrera, D.E.; Karshalev, E.; Nguyen, B.; Fang, R.H.; Zhang, L.; Wang, J. Multicompartment Tubular Micromotors Toward Enhanced Localized Active Delivery. Adv. Mater. 2020, 32, 2000091. [Google Scholar] [CrossRef]
- Nourhani, A.; Karshalev, E.; Soto, F.; Wang, J. Multigear Bubble Propulsion of Transient Micromotors. Research 2020, 2020, 7823615. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, Q.; Li, S.; Wang, X.; Han, X. Bowl-like Micromotors Using Red Blood Cell Membrane as Template. ChemistrySelect 2019, 4, 10296–10298. [Google Scholar] [CrossRef]
- Gregory, D.A.; Campbell, A.I.; Ebbens, S.J. Effect of Catalyst Distribution on Spherical Bubble Swimmer Trajectories. J. Phys. Chem. C 2015, 119, 15339–15348. [Google Scholar] [CrossRef]
- Adams, L.L.A.; Lee, D.; Mei, Y.; Weitz, D.A.; Solovev, A.A. Nanoparticle-Shelled Catalytic Bubble Micromotor. Adv. Mater. Interfaces 2020, 7, 1901583. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, T.; Zheng, M.; Jiang, Z.; Wang, S. Controlled one-sided growth of Janus TiO2/MnO2 nanomotors. Nanotechnology 2019, 30, 315702. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Wu, J.; Ju, H. Bubble-Propelled Jellyfish-like Micromotors for DNA Sensing. ACS Appl. Mater. Interfaces 2019, 11, 13581–13588. [Google Scholar] [CrossRef]
- Wang, J.; Toebes, B.J.; Plachokova, A.S.; Liu, Q.; Deng, D. Self-Propelled PLGA Micromotor with Chemotactic Response to Inflammation. Adv. Healthc. Mater. 2020, 9, 1901710. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Xie, S.; Liu, Y.; Liu, Q.; Song, X.; Li, X. Janus micromotors for motion-capture-lighting of bacteria. Nanoscale 2019, 11, 17831–17840. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Mestdagh, M.M.; Rouxhet, P.G. Reversible inhibition and irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1996, 1295, 222–238. [Google Scholar] [CrossRef]
- Simmchen, J.; Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Improving catalase-based propelled motor endurance by enzyme encapsulation. Nanoscale 2014, 6, 8907–8913. [Google Scholar] [CrossRef]
- Ren, M.; Guo, W.; Guo, H.; Ren, X. Microfluidic Fabrication of Bubble-Propelled Micromotors for Wastewater Treatment. ACS Appl. Mater. Interfaces 2019, 11, 22761–22767. [Google Scholar] [CrossRef]
- Si, T.; Zou, X.; Wu, Z.; Li, T.; Wang, X.; Ivanovich, K.I.; He, Q. A Bubble-Dragged Catalytic Polymer Microrocket. Chem.-Asian J. 2019, 14, 2460–2464. [Google Scholar] [CrossRef]
- Chen, C.; He, Z.; Wu, J.; Zhang, X.; Xia, Q.; Ju, H. Motion of Enzyme-Powered Microshell Motors. Chem.-Asian J. 2019, 14, 2491–2496. [Google Scholar] [CrossRef]
- Keller, S.; Teora, S.P.; Hu, G.X.; Nijemeisland, M.; Wilson, D.A. High-Throughput Design of Biocompatible Enzyme-Based Hydrogel Microparticles with Autonomous Movement. Angew. Chem. Int. Ed. 2018, 57, 9814–9817. [Google Scholar] [CrossRef]
- Costa, S.A.; Tzanov, T.; Filipa Carneiro, A.; Paar, A.; Gübitz, G.M.; Cavaco-Paulo, A. Studies of stabilization of native catalase using additives. Enzym. Microb. Technol. 2002, 30, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef] [Green Version]
- Belluzo, S.; Boeris, V.; Farruggia, B.; Picó, G. Influence of stabilizers cosolutes on catalase conformation. Int. J. Biol. Macromol. 2011, 49, 936–941. [Google Scholar] [CrossRef]
- Altikatoglu, M.; Arioz, C.; Basaran, Y.; Kuzu, H. Stabilization of horseradish peroxidase by covalent conjugation with dextran aldehyde against temperature and pH changes. Open Chem. 2009, 7, 423. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, S.; Teora, S.P.; Keskin, A.; Daris, L.J.C.; Samuels, N.A.P.E.; Boujemaa, M.; Wilson, D.A. Spatial Control over Catalyst Positioning for Increased Micromotor Efficiency. Gels 2023, 9, 164. https://doi.org/10.3390/gels9020164
Keller S, Teora SP, Keskin A, Daris LJC, Samuels NAPE, Boujemaa M, Wilson DA. Spatial Control over Catalyst Positioning for Increased Micromotor Efficiency. Gels. 2023; 9(2):164. https://doi.org/10.3390/gels9020164
Chicago/Turabian StyleKeller, Shauni, Serena P. Teora, Arif Keskin, Luuk J. C. Daris, Norman A. P. E. Samuels, Moussa Boujemaa, and Daniela A. Wilson. 2023. "Spatial Control over Catalyst Positioning for Increased Micromotor Efficiency" Gels 9, no. 2: 164. https://doi.org/10.3390/gels9020164