Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery
Abstract
:1. Introduction
2. Drug Delivery Using Hydrogel Nanocomposites
2.1. Formulation Methods of Peptide-Hydrogel Nanocomposites
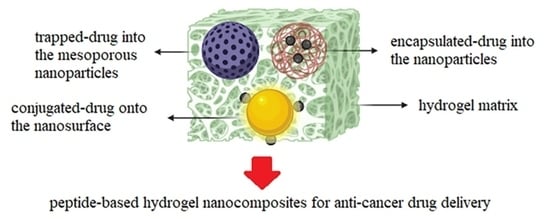
- Passively controlled drug release: Drug molecules in the hydrogel matrix are passively released through swelling and diffusion.
- Stimuli-responsive drug delivery: Smart hydrogels drastically change their volume in response to environmental stimuli (e.g., pH, temperature, magnetic field, light, and chemical signals), triggering the release of the loaded drug.
- Site-specific drug delivery: Drug delivery primarily relies on the properties of the hydrogel network comprising targeting components (e.g., antibodies, aptamer, folate, peptides), which allow nanoparticle design to be only focused on tuning the drug release. Such a hybrid strategy provides an opportunity to design highly specific DDSs [53].
2.2. Peptide-Based Hydrogel Nanocomposites Containing Inorganic NPs
2.3. Peptide-Based Hydrogel Nanocomposites Containing Organic NPs
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Voycheva, C.; Slavkova, M.; Popova, T.; Tzankova, D.; Stefanova, D.; Tzankova, V.; Ivanova, I.; Tzankov, S.; Spassova, I.; Kovacheva, D.; et al. Thermosensitive Hydrogel-Functionalized Mesoporous Silica Nanoparticles for Parenteral Application of Chemotherapeutics. Gels 2023, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological Advancements in Cancer Diagnostics: Improvements and Limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef] [PubMed]
- Cerra, S.; Dini, V.; Salamone, T.A.; Hajareh Haghighi, F.; Mercurio, M.; Cartoni, A.; Del Giudice, A.; Marsotto, M.; Venditti, I.; Battocchio, C.; et al. Acrylates-Based Hydrophilic Co-Polymeric Nanobeads as Nanocarriers for Imaging Agents. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131829. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Wu, W.; Banerjee, P.; Zhou, S. Biocompatible Anisole-Nonlinear PEG Core–Shell Nanogels for High Loading Capacity, Excellent Stability, and Controlled Release of Curcumin. Gels 2023, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled Nanomaterial for Cancer Diagnostics and Therapeutics: Principles and Concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Jackson, N.; Eaton, S.; Santos, N.D.; Barta, I.; Zaifman, J.; Chen, S.; Tam, Y.Y.C.; Krishnan, S.; Chithrani, D.B. Lipid-Nanoparticle-Mediated Delivery of Docetaxel Prodrug for Exploiting Full Potential of Gold Nanoparticles in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 6137. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Walker, E.; Springer, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Chemoradiotherapy of Prostate Cancer Using Gold Nanoclusters with Protease Activatable Monomethyl Auristatin E. ACS Appl. Mater. Interfaces 2022, 14, 14916–14927. [Google Scholar] [CrossRef] [PubMed]
- Elballa, W.; Schwinghamer, K.; Ebert, E.; Siahaan, T.J. Peptides and Their Delivery to the Brain BT—Peptide Therapeutics: Fundamentals of Design, Development, and Delivery; Jois, S.D., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 237–266. ISBN 978-3-031-04544-8. [Google Scholar]
- Kargari Aghmiouni, D.; Khoee, S. Dual-Drug Delivery by Anisotropic and Uniform Hybrid Nanostructures: A Comparative Study of the Function and Substrate–Drug Interaction Properties. Pharmaceutics 2023, 15, 1214. [Google Scholar]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Asad, S.; Jacobsen, A.-C.; Teleki, A. Inorganic Nanoparticles for Oral Drug Delivery: Opportunities, Barriers, and Future Perspectives. Curr. Opin. Chem. Eng. 2022, 38, 100869. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update Post COVID-19 Vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef] [PubMed]
- Khosravian, P.; Shafiee Ardestani, M.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Akbari Javar, H.; Amanlou, M. Mesoporous Silica Nanoparticles Functionalized with Folic Acid/Methionine for Active Targeted Delivery of Docetaxel. Onco. Targets Ther. 2016, 9, 7315–7330. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA Coated Iron Oxide Nanoparticles as Drug Delivery Vehicles for Cancer Therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.; Goyal, N.; Gupta, S. A recipe for optimizing TiO2 nanoparticles for drug delivery applications. OpenNano 2022, 8, 100096. [Google Scholar] [CrossRef]
- Hussein, H.A.; Abdullah, M.A. Novel Drug Delivery Systems Based on Silver Nanoparticles, Hyaluronic Acid, Lipid Nanoparticles and Liposomes for Cancer Treatment. Appl. Nanosci. 2022, 12, 3071–3096. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Koushki, K.; Keshavarz Shahbaz, S.; Keshavarz, M.; Bezsonov, E.E.; Sathyapalan, T.; Sahebkar, A. Gold Nanoparticles: Multifaceted Roles in the Management of Autoimmune Disorders. Biomolecules 2021, 11, 1289. [Google Scholar] [CrossRef]
- Gerosa, C.; Crisponi, G.; Nurchi, V.M.; Saba, L.; Cappai, R.; Cau, F.; Faa, G.; Van Eyken, P.; Scartozzi, M.; Floris, G.; et al. Gold Nanoparticles: A New Golden Era in Oncology? Pharmaceuticals 2020, 13, 192. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, G.; Luo, Z.; Zhou, L.; Xue, Y.; Liu, M. Recent Progress in the Applications of Gold-Based Nanoparticles towards Tumor-Targeted Imaging and Therapy. RSC Adv. 2022, 12, 7635–7651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, D.; Zhang, C.; Liu, H.; Hao, M.; Kan, S.; Liu, D.; Liu, W. The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front. Oncol. 2022, 11, 819329. [Google Scholar] [CrossRef] [PubMed]
- Kaphle, A.; Jayarathna, S.; Moktan, H.; Aliru, M.; Raghuram, S.; Krishnan, S.; Cho, S.H. Deep Learning-Based TEM Image Analysis for Fully Automated Detection of Gold Nanoparticles Internalized Within Tumor Cell. Microsc. Microanal. 2023, 29, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Hill, I.; Alhussan, A.; Bromma, K.; Morgan, J.; Abousaida, B.; Zahra, Y.; Mackeyev, Y.; Beckham, W.; Herchko, S.; et al. Dual Enhancement in the Radiosensitivity of Prostate Cancer through Nanoparticles and Chemotherapeutics. Cancer Nanotechnol. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, S.; Mackeyev, Y.; Symons, J.; Zahra, Y.; Gonzalez, V.; Mahadevan, K.K.; Requejo, K.I.; Liopo, A.; Derry, P.; Zubarev, E.; et al. Uncloaking Cell-Impermeant Gold Nanorods via Tumor Microenvironmental Cathepsin B Facilitates Cancer Cell Penetration and Potent Radiosensitization. Biomaterials 2022, 291, 121887. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Mackeyev, Y.; Sanders, K.; Kim, J.B.K.; Gonzalez, V.V.; Zahra, Y.; Shohayeb, M.A.; Abousaida, B.; Vijay, G.V.; Tezcan, O.; et al. Pancreatic Tumor Microenvironmental Acidosis and Hypoxia Transform Gold Nanorods into Cell-Penetrant Particles for Potent Radiosensitization. Sci. Adv. 2023, 8, eabm9729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Man, T.; Xu, X.; Yang, Q.; Liu, W.; Jonas, S.J.; Teitell, M.A.; Chiou, P.-Y.; Weiss, P.S. Photothermal Intracellular Delivery Using Gold Nanodisk Arrays. ACS Mater. Lett. 2020, 2, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Peters, G.J.; Ossendorp, F.; Cruz, L.J. The Potential of Multi-Compound Nanoparticles to Bypass Drug Resistance in Cancer. Cancer Chemother. Pharmacol. 2017, 80, 881–894. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Binaymotlagh, R.; Cerra, S.; Haghighi, F.H.; Di Domenico, E.G.; Sivori, F.; Fratoddi, I.; Mignardi, S.; Palocci, C. Preparation of Hydrogel Composites Using a Sustainable Approach for in situ Silver Nanoparticles Formation. Materials 2023, 16, 2134. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green in situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ Sprayed Bioresponsive Immunotherapeutic Gel for Post-Surgical Cancer Treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wan, K.; Zhou, N.; Wei, G.; Su, Z. Supramolecular Peptide Nano-Assemblies for Cancer Diagnosis and Therapy: From Molecular Design to Material Synthesis and Function-Specific Applications. J. Nanobiotechnol. 2021, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Choi, Y.J.; Jang, M.-S.; Lee, J.H.; Jeong, J.H.; Kim, J. Supertough Hybrid Hydrogels Consisting of a Polymer Double-Network and Mesoporous Silica Microrods for Mechanically Stimulated On-Demand Drug Delivery. Adv. Funct. Mater. 2017, 27, 1703826. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Liu, C.; Guo, X.; Ruan, C.; Hu, H.; Jiang, B.-P.; Liang, H.; Shen, X.-C. An Injectable Thermosensitive Photothermal-Network Hydrogel for near-Infrared-Triggered Drug Delivery and Synergistic Photothermal-Chemotherapy. Acta Biomater. 2019, 96, 281–294. [Google Scholar] [CrossRef]
- Wu, H.; Song, L.; Chen, L.; Zhang, W.; Chen, Y.; Zang, F.; Chen, H.; Ma, M.; Gu, N.; Zhang, Y. Injectable Magnetic Supramolecular Hydrogel with Magnetocaloric Liquid-Conformal Property Prevents Post-Operative Recurrence in a Breast Cancer Model. Acta Biomater. 2018, 74, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Huo, S.; Wang, T.; Sun, W.; Tong, Z. Self-Healable Tough Supramolecular Hydrogels Crosslinked by Poly-Cyclodextrin through Host-Guest Interaction. Carbohydr. Polym. 2018, 193, 54–61. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.R.; Appel, E.A.; Seitsonen, J.; Kontturi, E.; Scherman, O.A.; Ikkala, O. Healable, Stable and Stiff Hydrogels: Combining Conflicting Properties Using Dynamic and Selective Three-Component Recognition with Reinforcing Cellulose Nanorods. Adv. Funct. Mater. 2014, 24, 2706–2713. [Google Scholar] [CrossRef]
- Servant, A.; Methven, L.; Williams, R.P.; Kostarelos, K. Electroresponsive Polymer-Carbon Nanotube Hydrogel Hybrids for Pulsatile Drug Delivery in vivo. Adv. Healthc. Mater. 2013, 2, 806–811. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-Sensitive Behavior of Intelligent Ferrogels for Controlled Release of Drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef]
- Cheng, Z.; Chai, R.; Ma, P.; Dai, Y.; Kang, X.; Lian, H.; Hou, Z.; Li, C.; Lin, J. Multiwalled Carbon Nanotubes and NaYF4:Yb3+/Er3+ Nanoparticle-Doped Bilayer Hydrogel for Concurrent NIR-Triggered Drug Release and Up-Conversion Luminescence Tagging. Langmuir 2013, 29, 9573–9580. [Google Scholar] [CrossRef] [PubMed]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Chronopoulou, L.; Cerra, S.; Marrani, A.G.; Amato, F.; Palocci, C.; Fratoddi, I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels 2023, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Veloso, S.R.S.; Correa-Duarte, M.A.; Ferreira, P.M.T.; Castanheira, E.M.S. Tuning Peptide-Based Hydrogels: Co-Assembly with Composites Driving the Highway to Technological Applications. Int. J. Mol. Sci. 2023, 24, 186. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Andrade, R.G.D.; Castanheira, E.M.S. Review on the Advancements of Magnetic Gels: Towards Multifunctional Magnetic Liposome-Hydrogel Composites for Biomedical Applications. Adv. Colloid Interface Sci. 2021, 288, 102351. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Xu, X.; Fan, Y. KRAS-Mutant Non-Small Cell Lung Cancer: An Emerging Promisingly Treatable Subgroup. Front. Oncol. 2021, 11, 672612. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, B.; Zhou, Z.; Meng, L.; Sun, Z.; Xu, Y.; Xu, Q.; Yuan, A.; Yu, L.; Qian, H.; et al. Enhancement of Radiotherapy Efficacy by Pleiotropic Liposomes Encapsulated Paclitaxel and Perfluorotributylamine. Drug Deliv. 2017, 24, 1419–1428. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Zhao, W.; Li, L.; Ye, J.; Wu, C.; Tang, H.; Lin, Q.; Li, J.; Xia, Y.; et al. A Randomized Phase 3 Trial Comparing Paclitaxel plus 5-Fluorouracil versus Cisplatin plus 5-Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Carcinoma—The ESO-Shanghai 1 Trial Protocol. Radiat. Oncol. 2018, 13, 33. [Google Scholar] [CrossRef]
- Patel, K.; Doddapaneni, R.; Sekar, V.; Chowdhury, N.; Singh, M. Combination Approach of YSA Peptide Anchored Docetaxel Stealth Liposomes with Oral Antifibrotic Agent for the Treatment of Lung Cancer. Mol. Pharm. 2016, 13, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-H.; Wang, Y.; Huang, D.; Wang, Y.; Shin, H.J.; Chen, Z.; Spewak, M.B.; Mao, H.; Wang, X.; Wang, Y.; et al. Targeted Delivery of Cisplatin to Lung Cancer Using ScFvEGFR-Heparin-Cisplatin Nanoparticles. ACS Nano 2011, 5, 9480–9493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fu, X.; Ou, Q.; Gao, K.; Man, S.-Q.; Guo, J.; Liu, Y. Hydroxide Assisted Synthesis of Monodisperse and Biocompatible Gold Nanoparticles with Dextran. Mater. Sci. Eng. C 2018, 93, 759–767. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Quintas, A.; Pérez-Núñez, D.; Nogal, M.; Barroso, S.; Carrascosa, Á.L.; Revilla, Y. African Swine Fever Virus Uses Macropinocytosis to Enter Host Cells. PLOS Pathog. 2012, 8, e1002754. [Google Scholar] [CrossRef]
- Janczewska, M.; Szkop, M.; Pikus, G.; Kopyra, K.; Świątkowska, A.; Brygoła, K.; Karczmarczyk, U.; Walczak, J.; Żuk, M.T.; Duszak, J.; et al. PSMA Targeted Conjugates Based on Dextran. Appl. Radiat. Isot. 2021, 167, 109439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liao, H.; Pu, Q.; Ke, X.; Hu, X.; Ma, Y.; Luo, X.; Jiang, Q.; Gong, Y.; Wu, M.; et al. MiR-410 Induces Both Epithelial–Mesenchymal Transition and Radioresistance through Activation of the PI3K/MTOR Pathway in Non-Small Cell Lung Cancer. Signal Transduct. Target. Ther. 2020, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, C.; Zhou, Y.; Zhang, W.; Hu, X.; Chen, M.; Hui, H.; Guo, L.; Wu, C.; Zhou, J.; et al. P-Y/G@NHs Sensitizes Non-Small Cell Lung Cancer Cells to Radiotherapy via Blockage of the PI3K/AKT Signaling Pathway. Bioorg. Chem. 2023, 131, 106317. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.P.; Niehues, M.; Ravoo, B.J. Magneto-Responsive Hydrogels by Self-Assembly of Low Molecular Weight Peptides and Crosslinking with Iron Oxide Nanoparticles. Soft Matter 2021, 17, 2857–2864. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-Based Plasmonic Magnetogels: A Supramolecular Composite Nanosystem for Multimodal Cancer Therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef]
- Wang, W.; Han, R.; Tang, K.; Zhao, S.; Ding, C.; Luo, X. Biocompatible Peptide Hydrogels with Excellent Antibacterial and Catalytic Properties for Electrochemical Sensing Application. Anal. Chim. Acta 2021, 1154, 338295. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.-Y.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-Induced Peripheral Neurotoxicity: A Critical Analysis. CA. Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z.; et al. In situ Formed Reactive Oxygen Species–Responsive Scaffold with Gemcitabine and Checkpoint Inhibitor for Combination Therapy. Sci. Transl. Med. 2018, 10, eaan3682. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Van Laethem, J.-L. Combination or Single-Agent Chemotherapy as Adjuvant Treatment for Pancreatic Cancer? Lancet Oncol. 2019, 20, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent Advances of Cocktail Chemotherapy by Combination Drug Delivery Systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local Triple-Combination Therapy Results in Tumour Regression and Prevents Recurrence in a Colon Cancer Model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Lin, C.M.; Liu, Q.; Huang, L. Nanoformulations for Combination or Cascade Anticancer Therapy. Adv. Drug Deliv. Rev. 2017, 115, 3–22. [Google Scholar] [CrossRef]
- Albiges, L.; Choueiri, T.; Escudier, B.; Galsky, M.; George, D.; Hofmann, F.; Lam, T.; Motzer, R.; Mulders, P.; Porta, C.; et al. A Systematic Review of Sequencing and Combinations of Systemic Therapy in Metastatic Renal Cancer. Eur. Urol. 2015, 67, 100–110. [Google Scholar] [CrossRef]
- Pathak, R.K.; Dhar, S. A Nanoparticle Cocktail: Temporal Release of Predefined Drug Combinations. J. Am. Chem. Soc. 2015, 137, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Gibney, G.; Sullivan, R.J.; Sosman, J.A.; Slingluff, C.L.; Lawrence, D.P.; Logan, T.F.; Schuchter, L.M.; Nair, S.; Fecher, L.; et al. Sequential Administration of Nivolumab and Ipilimumab with a Planned Switch in Patients with Advanced Melanoma (CheckMate 064): An Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2016, 17, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Tan, D.S.W.; De Pas, T.; Solomon, B.J.; Ahmad, A.; Lazzari, C.; de Marinis, F.; Spitaleri, G.; Schultz, K.; Friboulet, L.; et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin. Cancer Res. 2015, 21, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Ye, A.S.; Gardino, A.K.; Heijink, A.M.; Sorger, P.K.; MacBeath, G.; Yaffe, M.B. Sequential Application of Anticancer Drugs Enhances Cell Death by Rewiring Apoptotic Signaling Networks. Cell 2012, 149, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Lim, H.-S.; Lee, D.H.; Ju, S.Y.; Lee, S.Y.; Kim, H.Y.; Park, Y.-H.; Park, C.G.; Lee, J.S. Randomized Phase II Study of Two Opposite Administration Sequences of Irinotecan and Cisplatin in Patients with Advanced Nonsmall Cell Lung Carcinoma. Cancer 2006, 106, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Maliepaard, M.; Nooter, K.; Boersma, A.W.M.; Verweij, J.; Stoter, G.; Schellens, J.H.M. Synergistic Cytotoxicity of Cisplatin and Topotecan or SN-38 in a Panel of Eight Solid-Tumor Cell Lines in vitro. Cancer Chemother. Pharmacol. 1998, 41, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Crul, M.; van Waardenburg, R.C.A.; Beijnen, J.H.; Schellens, J.H.M. DNA-Based Drug Interactions of Cisplatin. Cancer Treat. Rev. 2002, 28, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Gazit, E. The Physical Properties of Supramolecular Peptide Assemblies: From Building Block Association to Technological Applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Ulijn, R. V Design of Nanostructures Based on Aromatic Peptide Amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide Supramolecular Materials for Therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, 1805798. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Zhang, P.; Lin, Y.; Lock, L.L.; Cui, H. Supramolecular Nanostructures Formed by Anticancer Drug Assembly. J. Am. Chem. Soc. 2013, 135, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shen, H.; Zhan, J.; Lin, M.; Dai, L.; Ren, C.; Shi, Y.; Liu, J.; Gao, J.; Yang, Z. Supramolecular “Trojan Horse” for Nuclear Delivery of Dual Anticancer Drugs. J. Am. Chem. Soc. 2017, 139, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, J.; Wan, J.; Geng, S.; Li, H.; Peng, X.; Fu, Q.; He, M.; Zhao, Y.; Yang, X. Cisplatin-Directed Coordination-Crosslinking Nanogels with Thermo/PH-Sensitive Triblock Polymers: Improvement on Chemotherapic Efficacy via Sustained Release and Drug Retention. Nanoscale 2017, 9, 5859–5871. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Liang, S.; Xu, S. Dual Stable Nanomedicines Prepared by Cisplatin-Crosslinked Camptothecin Prodrug Micelles for Effective Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 20649–20659. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, J.; Zhai, Z.; Yang, L.; Tang, X.; Zhao, L.; Xu, K.; Zhong, W. Double-Crosslinked Nanocomposite Hydrogels for Temporal Control of Drug Dosing in Combination Therapy. Acta Biomater. 2020, 106, 278–288. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, X.; Xu, X.; Sun, T. Influence of PH on the Self-Assembly of Diphenylalanine Peptides: Molecular Insights from Coarse-Grained Simulations. Soft Matter 2023, 19, 5749–5757. [Google Scholar] [CrossRef]
- Gazit, E. Self-Assembled Peptide Nanostructures: The Design of Molecular Building Blocks and Their Technological Utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef]
- Habibi, N.; Soumetz, F.C.; Giulianelli, M.; Pastorino, L.; Herrera, O.; Sbrana, F.; Raiteri, R.; Ruggiero, C. Self-Assembly and Recrystallization of Bacterial S-Layer Proteins of Bacillus Sphaericus and Bacillus Thuringiensis on Silicone, Mica and Quartz Crystal Supports. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3739–3742. [Google Scholar]
- Detzel, C.J.; Larkin, A.L.; Rajagopalan, P. Polyelectrolyte Multilayers in Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 101–113. [Google Scholar] [CrossRef]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-Assembled Peptide-Based Nanostructures: Smart Nanomaterials toward Targeted Drug Delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Self-Assembly of Peptides to Nanostructures. Org. Biomol. Chem. 2014, 12, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The Biocompatibility of Carbon Nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Designed Aromatic Homo-Dipeptides: Formation of Ordered Nanostructures and Potential Nanotechnological Applications. Phys. Biol. 2006, 3, S10. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Araújo, D.R.; Silva, E.R.; Ando, R.A.; Alves, W.A. L-Diphenylalanine Microtubes As a Potential Drug-Delivery System: Characterization, Release Kinetics, and Cytotoxicity. Langmuir 2013, 29, 10205–10212. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-Based Theranostic Agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Kalber, T.; Thanou, M.; Bell, J.D.; Miller, A.D. Folate Receptor Targeted Bimodal Liposomes for Tumor Magnetic Resonance Imaging. Bioconjug. Chem. 2009, 20, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Nie, L.; Li, F.; Aguilar, Z.P.; Xu, H.; Xiong, Y.; Fu, F.; Xu, H. Folic Acid Conjugated Magnetic Iron Oxide Nanoparticles for Nondestructive Separation and Detection of Ovarian Cancer Cells from Whole Blood. Biomater. Sci. 2016, 4, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-B.; Shin, H.; Garcia, A.J.; Bunz, U.H.F. Use of a Folate−PPE Conjugate To Image Cancer Cells in vitro. Bioconjug. Chem. 2007, 18, 815–820. [Google Scholar] [CrossRef]
- Sega, E.I.; Low, P.S. Tumor Detection Using Folate Receptor-Targeted Imaging Agents. Cancer Metastasis Rev. 2008, 27, 655–664. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Tegafaw, T.; Liu, S.; Ahmad, M.Y.; Saidi, A.K.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Magnetic Nanoparticle-Based High-Performance Positive and Negative Magnetic Resonance Imaging Contrast Agents. Pharmaceutics 2023, 15, 1745. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.L.; Zheng, S.W.; Hong, R.Y.; Deng, S.M.; Guo, L.; Hu, R.L.; Gao, B.; Huang, M.; Cheng, L.F.; Liu, G.H.; et al. Folic Acid-Conjugated Fe3O4 Magnetic Nanoparticles for Hyperthermia and MRI in vitro and in vivo. Appl. Surf. Sci. 2014, 307, 224–233. [Google Scholar] [CrossRef]
- Honarmand, D.; Ghoreishi, S.M.; Habibi, N.; Nicknejad, E.T. Controlled Release of Protein from Magnetite–Chitosan Nanoparticles Exposed to an Alternating Magnetic Field. J. Appl. Polym. Sci. 2016, 133, 43335. [Google Scholar] [CrossRef]
- Castillo, J.J.; Rindzevicius, T.; Novoa, L.V.; Svendsen, W.E.; Rozlosnik, N.; Boisen, A.; Escobar, P.; Martínez, F.; Castillo-León, J. Non-Covalent Conjugates of Single-Walled Carbon Nanotubes and Folic Acid for Interaction with Cells over-Expressing Folate Receptors. J. Mater. Chem. B 2013, 1, 1475–1481. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, J.; Lai, Y.; Ma, Y.; Weng, J.; Sun, L. Conjugating Folic Acid to Gold Nanoparticles through Glutathione for Targeting and Detecting Cancer Cells. Bioorg. Med. Chem. 2010, 18, 5528–5534. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Low, P.S. Delivery of Liposomes into Cultured KB Cells via Folate Receptor-Mediated Endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing Ferumoxytol: Diagnostic and Therapeutic Applications of an FDA-Approved Nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef] [PubMed]
- Emtiazi, G.; Zohrabi, T.; Lee, L.Y.; Habibi, N.; Zarrabi, A. Covalent Diphenylalanine Peptide Nanotube Conjugated to Folic Acid/Magnetic Nanoparticles for Anti-Cancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 90–98. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Kartal-Yandim, M.; Adan-Gokbulut, A.; Baran, Y. Molecular Mechanisms of Drug Resistance and Its Reversal in Cancer. Crit. Rev. Biotechnol. 2016, 36, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Resnier, P.; Montier, T.; Mathieu, V.; Benoit, J.-P.; Passirani, C. A Review of the Current Status of SiRNA Nanomedicines in the Treatment of Cancer. Biomaterials 2013, 34, 6429–6443. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery Materials for SiRNA Therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable Hydrogels for Delivering Biotherapeutic Molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.H.; Ruiz, R.C.H.; Peng, S.; Lee, J.B.; Luo, D. Engineering DNA-Based Functional Materials. Chem. Soc. Rev. 2011, 40, 5730–5744. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef]
- Chen, L.-H.; Liang, N.-W.; Huang, W.-Y.; Liu, Y.-C.; Ho, C.-Y.; Kuan, C.-H.; Huang, Y.-F.; Wang, T.-W. Supramolecular Hydrogel for Programmable Delivery of Therapeutics to Cancer Multidrug Resistance. Biomater. Adv. 2023, 146, 213282. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Marmiroli, P. Chemotherapy-Induced Peripheral Neurotoxicity in the Era of Pharmacogenomics. Lancet Oncol. 2011, 12, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalt. Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Herrmann, J. Vascular Toxic Effects of Cancer Therapies. Nat. Rev. Cardiol. 2020, 17, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Huang, W.-T.; Liu, D.-M.; Losic, D. Local Co-Administration of Gene-Silencing RNA and Drugs in Cancer Therapy: State-of-the Art and Therapeutic Potential. Cancer Treat. Rev. 2017, 55, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, 1801183. [Google Scholar] [CrossRef]
- Ji, T.; Kohane, D.S. Nanoscale Systems for Local Drug Delivery. Nano Today 2019, 28, 100765. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable Thermosensitive Hydrogel Systems Based on Functional PEG/PCL Block Polymer for Local Drug Delivery. J. Control. Release 2019, 297, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Zhang, C.; Shih, T.-Y.; Xu, Q.; Schuster, B.S.; Hanes, J. Brain-Penetrating Nanoparticles Improve Paclitaxel Efficacy in Malignant Glioma Following Local Administration. ACS Nano 2014, 8, 10655–10664. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.T.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A.Z. Co-Delivery of Paclitaxel and Cisplatin with Biocompatible PLGA–PEG Nanoparticles Enhances Chemoradiotherapy in Non-Small Cell Lung Cancer Models. J. Mater. Chem. B 2017, 5, 6049–6057. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hou, Y.-F.; Niu, P.-F.; Zhang, K.; Shoji, T.; Tsuboi, Y.; Yao, F.-Y.; Zhao, L.-M.; Chang, J.-B. Biodegradable PLGA Nanoparticles Loaded with Hydrophobic Drugs: Confocal Raman Microspectroscopic Characterization. J. Mater. Chem. B 2015, 3, 3677–3680. [Google Scholar] [CrossRef]
- Štaka, I.; Cadete, A.; Surikutchi, B.T.; Abuzaid, H.; Bradshaw, T.D.; Alonso, M.J.; Marlow, M. A Novel Low Molecular Weight Nanocomposite Hydrogel Formulation for Intra-Tumoural Delivery of Anti-Cancer Drugs. Int. J. Pharm. 2019, 565, 151–161. [Google Scholar] [CrossRef]
- Unterman, S.; Charles, L.F.; Strecker, S.E.; Kramarenko, D.; Pivovarchik, D.; Edelman, E.R.; Artzi, N. Hydrogel Nanocomposites with Independently Tunable Rheology and Mechanics. ACS Nano 2017, 11, 2598–2610. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, Y.; Xue, X.; Zhou, S.; Li, C.; Zhang, X.; Jiang, X. Entrapping Multifunctional Dendritic Nanoparticles into a Hydrogel for Local Therapeutic Delivery and Synergetic Immunochemotherapy. Nano Res. 2018, 11, 6062–6073. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Huang, Z.; Zhang, X.; He, S.; Sun, X.; Shen, Y.; Yan, M.; Zhao, C. Injectable, NIR/PH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 20361–20375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An Injectable, Thermosensitive and Multicompartment Hydrogel for Simultaneous Encapsulation and Independent Release of a Drug Cocktail as an Effective Combination Therapy Platform. J. Control. Release 2015, 203, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, A.; Bai, Y.; Kong, D.; Lv, F. Dual Fluorescence Imaging-Guided Programmed Delivery of Doxorubicin and CpG Nanoparticles to Modulate Tumor Microenvironment for Effective Chemo-Immunotherapy. Biomaterials 2020, 230, 119659. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Mizutani, Y.; Nonomura, N.; Nomoto, T.; Nakao, M.; Saiki, S.; Kotake, T.; Okuyama, A. Irinotecan plus Cisplatin Has Substantial Antitumor Effect as Salvage Chemotherapy against Germ Cell Tumors. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2002, 95, 1879–1885. [Google Scholar] [CrossRef]
- Morris, P.G.; Oda, J.; Heinemann, M.-H.; Ilson, D.H. Choroidal Metastases From Esophageal Adenocarcinoma Responding to Chemotherapy With Cisplatin and Irinotecan. J. Clin. Oncol. 2010, 28, e372–e373. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Han, J.-Y.; Moon, S.H.; Nam, B.-H.; Lim, K.Y.; Lee, G.K.; Kim, H.T.; Yun, T.; An, H.J.; Lee, J.S. Incorporating Erlotinib or Irinotecan Plus Cisplatin into Chemoradiotherapy for Stage III Non-Small Cell Lung Cancer According to EGFR Mutation Status. Cancer Res. Treat. 2017, 49, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, C.; Sun, L.; Xu, K.; Zhong, W. PLGA Nanoparticle-Reinforced Supramolecular Peptide Hydrogels for Local Delivery of Multiple Drugs with Enhanced Synergism. Soft Matter 2020, 16, 10528–10536. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Sobrero, A.; Carnaghi, C.; Comella, P.; Díaz-Rubio, E.; Santoro, A.; Van Cutsem, E. Metastatic Colorectal Cancer: Integrating Irinotecan into Combination and Sequential Chemotherapy. Ann. Oncol. 2003, 14, ii7–ii12. [Google Scholar] [CrossRef] [PubMed]
- Simkens, L.H.J.; van Tinteren, H.; May, A.; ten Tije, A.J.; Creemers, G.-J.M.; Loosveld, O.J.L.; de Jongh, F.E.; Erdkamp, F.L.G.; Erjavec, Z.; van der Torren, A.M.E.; et al. Maintenance Treatment with Capecitabine and Bevacizumab in Metastatic Colorectal Cancer (CAIRO3): A Phase 3 Randomised Controlled Trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Malka, D.; Mendiboure, J.; Etienne, P.-L.; Texereau, P.; Auby, D.; Rougier, P.; Gasmi, M.; Castaing, M.; Abbas, M.; et al. Sequential versus Combination Chemotherapy for the Treatment of Advanced Colorectal Cancer (FFCD 2000–05): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2011, 12, 1032–1044. [Google Scholar] [CrossRef]
- Cardoso, F.; Bedard, P.L.; Winer, E.P.; Pagani, O.; Senkus-Konefka, E.; Fallowfield, L.J.; Kyriakides, S.; Costa, A.; Cufer, T.; Albain, K.S.; et al. International Guidelines for Management of Metastatic Breast Cancer: Combination vs Sequential Single-Agent Chemotherapy. JNCI J. Natl. Cancer Inst. 2009, 101, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing Biocompatibility of Graphene Oxide-Based Nanocarriers: A Review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A Boon to Drug Delivery, Therapeutics, Diagnostics and Imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Anand Subramony, J. Engineered In-Situ Depot-Forming Hydrogels for Intratumoral Drug Delivery. J. Control. Release 2015, 220, 465–475. [Google Scholar] [CrossRef]
- Shen, B.; Lu, D.; Zhai, W.; Zheng, W. Synthesis of Graphene by Low-Temperature Exfoliation and Reduction of Graphite Oxide under Ambient Atmosphere. J. Mater. Chem. C 2013, 1, 50–53. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin Loaded Graphene Oxide Nanoparticle Functionalized with Polyethylene Glycol and Folic Acid for Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Deb, A.; Andrews, N.G.; Raghavan, V. Natural Polymer Functionalized Graphene Oxide for Co-Delivery of Anticancer Drugs: In-Vitro and in-Vivo. Int. J. Biol. Macromol. 2018, 113, 515–525. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Development of a Graphene Oxide Nanocarrier for Dual-Drug Chemo-Phototherapy to Overcome Drug Resistance in Cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Lu, Y.-J.; Chen, J.-P. Magnetic Graphene Oxide as a Carrier for Targeted Delivery of Chemotherapy Drugs in Cancer Therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Jabbehdari, S.; Omidi, M.; Tayebi, L. Functionalized R9–Reduced Graphene Oxide as an Efficient Nano-Carrier for Hydrophobic Drug Delivery. RSC Adv. 2016, 6, 74072–74084. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, S.; Wang, M.; Li, Y.; Shi, P.; Huang, X. Delivery of Paclitaxel Using PEGylated Graphene Oxide as a Nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Tao, L.; Annie Bligh, S.W.; Yang, H.; Pan, Q.; Zhu, L. Targeted Delivery and Controlled Release of Doxorubicin into Cancer Cells Using a Multifunctional Graphene Oxide. Mater. Sci. Eng. C 2016, 59, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Liu, Z. Stimuli Responsive Drug Delivery Systems Based on Nano-Graphene for Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced Review of Graphene-Based Nanomaterials in Drug Delivery Systems: Synthesis, Modification, Toxicity and Application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Dexter, A.F.; Fletcher, N.L.; Creasey, R.G.; Filardo, F.; Boehm, M.W.; Jack, K.S. Fabrication and Characterization of Hydrogels Formed from Designer Coiled-Coil Fibril-Forming Peptides. RSC Adv. 2017, 7, 27260–27271. [Google Scholar] [CrossRef]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling Hydrogelation Kinetics by Peptide Design for Three-Dimensional Encapsulation and Injectable Delivery of Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.C.; Pochan, D.J.; Wagner, N.J.; Schneider, J.P. Macromolecular Diffusion and Release from Self-Assembled β-Hairpin Peptide Hydrogels. Biomaterials 2009, 30, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. An Injectable Nanocomposite Hydrogel Co-Constructed with Gold Nanorods and Paclitaxel-Loaded Nanoparticles for Local Chemo-Photothermal Synergetic Cancer Therapy. J. Mater. Chem. B 2019, 7, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Bucatariu, S.-M.; Doroftei, F.; Fundueanu, G. Smart Composite Materials Based on Chitosan Microspheres Embedded in Thermosensitive Hydrogel for Controlled Delivery of Drugs. Carbohydr. Polym. 2017, 157, 493–502. [Google Scholar] [CrossRef]
- Lammers, T.; Subr, V.; Ulbrich, K.; Peschke, P.; Huber, P.E.; Hennink, W.E.; Storm, G. Simultaneous Delivery of Doxorubicin and Gemcitabine to Tumors in vivo Using Prototypic Polymeric Drug Carriers. Biomaterials 2009, 30, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, G.; Liu, G.; Hu, J.; Liu, S. Photo- and Thermo-Responsive Multicompartment Hydrogels for Synergistic Delivery of Gemcitabine and Doxorubicin. J. Control. Release 2017, 259, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.; Feng, X.; Deng, M.; Xie, G.; Wang, J.; Zhang, L.; Liu, Q.; Yuan, P. Micellar Nanoparticles Loaded with Gemcitabine and Doxorubicin Showed Synergistic Effect. Colloids Surf. B Biointerfaces 2014, 113, 158–168. [Google Scholar] [CrossRef]
- Vogus, D.R.; Evans, M.A.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.M.; et al. A Hyaluronic Acid Conjugate Engineered to Synergistically and Sequentially Deliver Gemcitabine and Doxorubicin to Treat Triple Negative Breast Cancer. J. Control. Release 2017, 267, 191–202. [Google Scholar] [CrossRef]
- Vogus, D.R.; Pusuluri, A.; Chen, R.; Mitragotri, S. Schedule Dependent Synergy of Gemcitabine and Doxorubicin: Improvement of in vitro Efficacy and Lack of in vitro-in vivo Correlation. Bioeng. Transl. Med. 2018, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Schneible, J.D.; Shi, K.; Young, A.T.; Ramesh, S.; He, N.; Dowdey, C.E.; Dubnansky, J.M.; Lilova, R.L.; Gao, W.; Santiso, E.; et al. Modified Gaphene Oxide (GO) Particles in Peptide Hydrogels: A Hybrid System Enabling Scheduled Delivery of Synergistic Combinations of Chemotherapeutics. J. Mater. Chem. B 2020, 8, 3852–3868. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Ruoslahti, E.; Meng, H. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano 2017, 11, 9567–9569. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kiran, A.V.V.V.; Kusuma Kumari, G.; Krishnamurthy, P.T.; Khaydarov, R.R. Tumor Microenvironment and Nanotherapeutics: Intruding the Tumor Fort. Biomater. Sci. 2021, 9, 7667–7704. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Ogawa, K.; Kato, N.; Kawakami, S. Recent Advances in Lipid Nanoparticles for Delivery of Nucleic Acid, MRNA, and Gene Editing-Based Therapeutics. Drug Metab. Pharmacokinet. 2022, 44, 100450. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled Release and Targeted Drug Delivery with Poly(Lactic-Co-Glycolic Acid) Nanoparticles: Reviewing Two Decades of Research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic Overview of Soft Materials as a Novel Frontier for MRI Contrast Agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef] [PubMed]
- Yee Kuen, C.; Masarudin, M.J. Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for Delivery, Imaging and Therapy. WIREs Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Nik, M.E.; Malaekeh-Nikouei, B.; Amin, M.; Hatamipour, M.; Teymouri, M.; Sadeghnia, H.R.; Iranshahi, M.; Jaafari, M.R. Liposomal Formulation of Galbanic Acid Improved Therapeutic Efficacy of Pegylated Liposomal Doxorubicin in Mouse Colon Carcinoma. Sci. Rep. 2019, 9, 9527. [Google Scholar] [CrossRef] [PubMed]
- Joniec, A.; Sek, S.; Krysinski, P. Magnetoliposomes as Potential Carriers of Doxorubicin to Tumours. Chem.-A Eur. J. 2016, 22, 17715–17724. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.H.; Zandieh-doulabi, B.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New Liposomal Doxorubicin Nanoformulation for Osteosarcoma: Drug Release Kinetic Study Based on Thermo and PH Sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Rosa, E.; Gallo, E.; Diaferia, C.; Morelli, G.; Stornaiuolo, M.; Accardo, A. Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Tei, L.; Botta, M.; Ravera, E.; Fragai, M.; Parigi, G.; Luchinat, C. 1H NMR Relaxometric Study of Chitosan-Based Nanogels Containing Mono- and Bis-Hydrated Gd(III) Chelates: Clues for MRI Probes of Improved Sensitivity. ACS Appl. Bio Mater. 2020, 3, 9065–9072. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Rosa, E.; Diaferia, C.; Gallo, E.; Morelli, G.; Accardo, A. Stable Formulations of Peptide-Based Nanogels. Molecules 2020, 25, 3455. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Accardo, A.; Morelli, G. Peptide-Based Hydrogels as Delivery Systems for Doxorubicin. J. Pept. Sci. 2022, 28, e3301. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Scheper, W.; Kelderman, S.; Fanchi, L.F.; Linnemann, C.; Bendle, G.; de Rooij, M.A.J.; Hirt, C.; Mezzadra, R.; Slagter, M.; Dijkstra, K.; et al. Low and Variable Tumor Reactivity of the Intratumoral TCR Repertoire in Human Cancers. Nat. Med. 2019, 25, 89–94. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nikolos, F.; Lee, Y.C.; Jain, A.; Tsouko, E.; Gao, H.; Kasabyan, A.; Leung, H.E.; Osipov, A.; Jung, S.Y.; et al. Tipping the Immunostimulatory and Inhibitory DAMP Balance to Harness Immunogenic Cell Death. Nat. Commun. 2020, 11, 6299. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Ang, B.; Xu, X.; Huang, X.; Wu, Y.; Sun, Y.; Wang, W.; Li, N.; Cao, X.; Wan, T. TLR4 Is Essential for Dendritic Cell Activation and Anti-Tumor T-Cell Response Enhancement by DAMPs Released from Chemically Stressed Cancer Cells. Cell. Mol. Immunol. 2014, 11, 150–159. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, W.; Ouyang, Y.; Liang, S.; Yi, Y.; Hao, H.; Yu, J.; Liu, Y.; Nie, Y.; Wang, T.; et al. ATP-Exhausted Nanocomplexes for Intratumoral Metabolic Intervention and Photoimmunotherapy. Biomaterials 2022, 284, 121503. [Google Scholar] [CrossRef]
- Jin, C.; Wang, Y.; Li, Y.; Li, J.; Zhou, S.; Yu, J.; Wang, Z.; Yu, Y.; Zhang, H.; Wang, D.; et al. Doxorubicin-Near Infrared Dye Conjugate Induces Immunogenic Cell Death to Enhance Cancer Immunotherapy. Int. J. Pharm. 2021, 607, 121027. [Google Scholar] [CrossRef]
- Jiang, M.; Zeng, J.; Zhao, L.; Zhang, M.; Ma, J.; Guan, X.; Zhang, W. Chemotherapeutic Drug-Induced Immunogenic Cell Death for Nanomedicine-Based Cancer Chemo–Immunotherapy. Nanoscale 2021, 13, 17218–17235. [Google Scholar] [CrossRef]
- Zheng, P.; Ding, B.; Jiang, Z.; Xu, W.; Li, G.; Ding, J.; Chen, X. Ultrasound-Augmented Mitochondrial Calcium Ion Overload by Calcium Nanomodulator to Induce Immunogenic Cell Death. Nano Lett. 2021, 21, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ma, S.; Zhu, X.; Chen, C.; Zhang, R.; Cao, Z.; Chen, X.; Zhang, L.; Zhu, Y.; Zhang, S.; et al. Versatile Ginsenoside Rg3 Liposomes Inhibit Tumor Metastasis by Capturing Circulating Tumor Cells and Destroying Metastatic Niches. Sci. Adv. 2023, 8, eabj1262. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qiao, Y.; Cao, J.; Ta, L.; Ci, T.; Ke, X. Biomimetic Doxorubicin/Ginsenoside Co-Loading Nanosystem for Chemoimmunotherapy of Acute Myeloid Leukemia. J. Nanobiotechnol. 2022, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Efimova, A.A.; Krasnikov, E.A.; Trosheva, K.S.; Popov, A.S.; Melik-Nubarov, N.S.; Krivtsov, G.G. Chitosan-Based Multi-Liposomal Complexes: Synthesis, Biodegradability and Cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, J.; Tian, X.; Hua, T.; Poon, T.; Koo, M.; Chan, W. Characteristics of Chitosan Fiber and Their Effects towards Improvement of Antibacterial Activity. Carbohydr. Polym. 2022, 280, 119031. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, Y.; Liu, N.; Liu, X.; Meng, T.; Cheng, B.; He, J.; You, J.; Yuan, H.; Hu, F. Selective Redox-Responsive Drug Release in Tumor Cells Mediated by Chitosan Based Glycolipid-like Nanocarrier. J. Control. Release 2015, 206, 91–100. [Google Scholar] [CrossRef]
- Yang, H.; Liu, T.; Xu, Y.; Su, G.; Liu, T.; Yu, Y.; Xu, B. Protein Corona Precoating on Redox-Responsive Chitosan-Based Nano-Carriers for Improving the Therapeutic Effect of Nucleic Acid Drugs. Carbohydr. Polym. 2021, 265, 118071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/Cisplatin Co-Loaded Hyaluronic Acid/Chitosan-Based Nanoparticles for in vitro Synergistic Combination Chemotherapy of Breast Cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, C.; Liu, N.; Zhou, X.; Chu, X.; Lv, F.; Gu, Y.; Yin, L.; Liu, J.; Zhou, J.; et al. The Programmed Site-Specific Delivery of LY3200882 and PD-L1 SiRNA Boosts Immunotherapy for Triple-Negative Breast Cancer by Remodeling Tumor Microenvironment. Biomaterials 2022, 284, 121518. [Google Scholar] [CrossRef]
- Jin, X.; Fu, Q.; Gu, Z.; Zhang, Z.; Lv, H. Injectable Corilagin/Low Molecular Weight Chitosan/PLGA-PEG-PLGA Thermosensitive Hydrogels for Localized Cancer Therapy and Promoting Drug Infiltration by Modulation of Tumor Microenvironment. Int. J. Pharm. 2020, 589, 119772. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. PH-Dependent Reversibly Activatable Cell-Penetrating Peptides Improve the Antitumor Effect of Artemisinin-Loaded Liposomes. J. Colloid Interface Sci. 2021, 586, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 20, 697. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef] [PubMed]
- Kishore Deb, P.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. Basic Fundam. Drug Deliv. 2019, 16, 651–684. [Google Scholar]
- Wu, H.; Wei, G.; Luo, L.; Li, L.; Gao, Y.; Tan, X.; Wang, S.; Chang, H.; Liu, Y.; Wei, Y.; et al. Ginsenoside Rg3 Nanoparticles with Permeation Enhancing Based Chitosan Derivatives Were Encapsulated with Doxorubicin by Thermosensitive Hydrogel and Anti-Cancer Evaluation of Peritumoral Hydrogel Injection Combined with PD-L1 Antibody. Biomater. Res. 2022, 26, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirzavi, F.; Barati, M.; Vakili-Ghartavol, R.; Roshan, M.K.; Mashreghi, M.; Soukhtanloo, M.; Jaafari, M.R. Pegylated Liposomal Encapsulation Improves the Antitumor Efficacy of Combretastatin A4 in Murine 4T1 Triple-Negative Breast Cancer Model. Int. J. Pharm. 2022, 613, 121396. [Google Scholar] [CrossRef]
- Guilbaud-Chéreau, C.; Dinesh, B.; Schurhammer, R.; Collin, D.; Bianco, A.; Ménard-Moyon, C. Protected Amino Acid-Based Hydrogels Incorporating Carbon Nanomaterials for Near-Infrared Irradiation-Triggered Drug Release. Appl. Mater. Interfaces 2019, 11, 13147–13157. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rasale, D.B.; Das, A.K. Blue Light Emitting Self-Healable Graphene Quantum Dots Embedded Hydrogels. RSC Adv. 2016, 6, 54793–54800. [Google Scholar] [CrossRef]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical Construction of a Mechanically Stable Peptide–Graphene Oxide Hybrid Hydrogel for Drug Delivery and Pulsatile Triggered Release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef]
- Li, B.; Criado-Gonzalez, M.; Adam, A.; Bizeau, J.; Mélart, C.; Carvalho, A.; Bégin, S.; Bégin, D.; Jierry, L.; Mertz, D. Peptide Hydrogels Assembled from Enzyme-Adsorbed Mesoporous Silica Nanostructures for Thermoresponsive Doxorubicin Release. ACS Appl. Nano Mater. 2022, 5, 120–125. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Fores, J.R.; Carvalho, A.; Blanck, C.; Schmutz, M.; Kocgozlu, L.; Schaaf, P.; Jierry, L.; Boulmedais, F. Phase Separation in Supramolecular Hydrogels Based on Peptide Self-Assembly from Enzyme-Coated Nanoparticles. Langmuir 2019, 35, 10838–10845. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene Oxide Containing Self-Assembling Peptide Hybrid Hydrogels as a Potential 3D Injectable Cell Delivery Platform for Intervertebral Disc Repair Applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Z.; Li, D.; Zhang, W.; Yu, X.; Liu, W.; Gong, C.; Wei, G.; Su, Z. Biomimetic Ultralight, Highly Porous, Shape-Adjustable, and Biocompatible 3D Graphene Minerals via Incorporation of Self-Assembled Peptide Nanosheets. Adv. Funct. Mater. 2018, 28, 1801056. [Google Scholar] [CrossRef]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Wei, G. Motif-Designed Peptide Nanofibers Decorated with Graphene Quantum Dots for Simultaneous Targeting and Imaging of Tumor Cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Li, C.; Ling, S.; Yang, X.; Chen, G.; Yang, Y.; Wang, Q. NIR-II Fluorescent Self-Assembled Peptide Nanochain for Ultrasensitive Detection of Peritoneal Metastasis. Angew. Chemie Int. Ed. 2019, 58, 11001–11006. [Google Scholar] [CrossRef]




| Benefits | Nanoparticles | Ref. |
|---|---|---|
| Controlled drug release under electrical stimuli | Carbon NPs | [38,45] |
| Controlled drug release under magnetic stimuli | Iron oxide magnetic NPs | [38,46] |
| Controlled drug release under light stimuli | Carbon NPs | [38,47] |
| Hydrogel-Inorganic Nanoparticle Composite | Advantages | Disadvantages |
|---|---|---|
| Iron oxide magnetic NPs [69,70,71] | Synergy with magnetic hyperthermia Magnetoresponse MRI contrast | Requires screening functionalization to achieve co-assembly |
| Gold/silver NPs [72] | Low-cost sensors Synthesis in situ Facile synthesis and tunability Synergy with photothermia /photodynamic therapy | Heterogeneous dispersion Uncontrolled release Challenging reproducibility of in situ synthesis |
| Hydrogel Nanocomposite | Application | Ref. |
|---|---|---|
| Oxidized carbon nanotubes GO + (Fmoc-Phe-OH/Fmoc-Tyr(Bzl)-OH Fmoc/Tyr-OH/Fmoc-Tyr(Bzl)-OH) hydrogel | drug delivery | [231] |
| Graphene quantum dots + (Amoc-Phe-OH Amoc-Tyr-OH) hydrogel | drug delivery | [232] |
| GO + (Py-Gly-Ala-Gly-Ala-Gly-Tyr-OH) hydrogel | drug delivery | [233] |
| Fmoc-FFpY-hydrogel assembled from enzyme-adsorbed mesoporous silica nanostructures | thermo-responsive doxorubicin release | [234] |
| Fmoc–FFpY hydrogel nanocomposite containing silica NPs functionalized covalently by alkaline phosphatase | N/A | [235] |
| graphene oxide as nano-filler for the reinforcement of FEFKFEFK (β-sheet forming self-assembling peptide) | intervertebral disc repair applications | [236] |
| self-assembling a motif-specific peptide molecule (LLVFGAKMLPHHGA) containing graphene foam | matrices for drug delivery or bone tissue engineering | [237] |
| RGDAEAKAEAKYWYAFAEAKAEAKRGD-hydrogel-graphene quantum dots | targeting and imaging of tumor cells | [238] |
| self-assembly of an amphiphilic peptide (APP) into a nanochain with subsequent chemical crosslinking of NIR-II Ag2S QDs | ultrasensitive Detection of Peritoneal Metastasis | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajareh Haghighi, F.; Binaymotlagh, R.; Fratoddi, I.; Chronopoulou, L.; Palocci, C. Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery. Gels 2023, 9, 953. https://doi.org/10.3390/gels9120953
Hajareh Haghighi F, Binaymotlagh R, Fratoddi I, Chronopoulou L, Palocci C. Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery. Gels. 2023; 9(12):953. https://doi.org/10.3390/gels9120953
Chicago/Turabian StyleHajareh Haghighi, Farid, Roya Binaymotlagh, Ilaria Fratoddi, Laura Chronopoulou, and Cleofe Palocci. 2023. "Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery" Gels 9, no. 12: 953. https://doi.org/10.3390/gels9120953