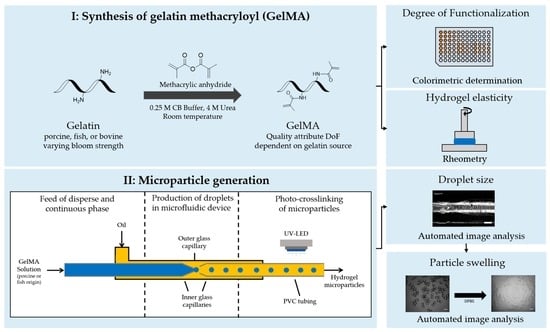
The Effect of Gelatin Source on the Synthesis of Gelatin-Methacryloyl and the Production of Hydrogel Microparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. GelMA Synthesis and Characterization
2.2. Hydrogel Characterization
2.3. Microparticle Generation and Characterization
3. Conclusions
4. Materials and Methods
4.1. Synthesis and Characterization of Gelatin-Methacryloyl
4.1.1. Precursor Solution for the Synthesis of Gelatin-Methacryloyl
4.1.2. Rheological Characterization of Gelatin Solutions
4.1.3. Synthesis and Purification
4.1.4. Determination of Degree of Functionalization
4.2. Hydrogel Characterization
4.2.1. Precursor Solution for the Production of Hydrogels
4.2.2. Mechanical Characterization
4.3. Microparticle Fabrication and Characterization
4.3.1. Precursor Solution for the Production of Microparticles
4.3.2. Rheological Characterization of Disperse and Continuous Phase
4.3.3. Microfluidic Production of Droplets and Crosslinking to Microparticles
4.3.4. Determination of Droplet Size
4.3.5. Determination of Hydrogel Swelling Behavior
4.4. Data Handling and Statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Carbonate-Bicarbonate |
| DoF | Degree of functionalization |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ECM | Extracellular matrix |
| GelMA | Gelatin-Methacryloyl |
| IEP | Isoelectric point |
| LED | Light-emitting diode |
| MAA | Methacrylic anhydride |
| MW | Molecular weight |
| RT | Room Temperature |
| TC | Tissue culture |
| TE | Tissue engineering |
| TNBS | 2,4,6-Trinitrobenzenesulfonic acid solution |
| UV | Ultraviolet |
| VSR | Volumetric swelling ratio |
References
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kunkel, J.; Asuri, P. Function, Structure, and Stability of Enzymes Confined in Agarose Gels. PLoS ONE 2014, 9, e86785. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; García-Carvajal, Z.Y.; Jobbágy, M.; Yuste, L.; Rojo, F.; Abrusci, C.; Catalina, F.; Del Monte, F.; Ferrer, M.L. Hydrogel scaffolds with immobilized bacteria for 3D cultures. Chem. Mater. 2007, 19, 1968–1973. [Google Scholar] [CrossRef]
- Takei, T.; Ikeda, K.; Ijima, H.; Kawakami, K. Fabrication of poly(vinyl alcohol) hydrogel beads crosslinked using sodium sulfate for microorganism immobilization. Process Biochem. 2011, 46, 566–571. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook; Wiley: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Shirahama, H.; Cho, N.J.; Tan, L.P. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed]
- Grijalva Garces, D.; Radtke, C.P.; Hubbuch, J. A Novel Approach for the Manufacturing of Gelatin-Methacryloyl. Polymers 2022, 14, 5424. [Google Scholar] [CrossRef] [PubMed]
- Aljaber, M.B.; Verisqa, F.; Keskin-Erdogan, Z.; Patel, K.D.; Chau, D.Y.; Knowles, J.C. Influence of Gelatin Source and Bloom Number on Gelatin Methacryloyl Hydrogels Mechanical and Biological Properties for Muscle Regeneration. Biomolecules 2023, 13, 811. [Google Scholar] [CrossRef]
- Sewald, L.; Claaßen, C.; Götz, T.; Claaßen, M.H.; Truffault, V.; Tovar, G.E.; Southan, A.; Borchers, K. Beyond the Modification Degree: Impact of Raw Material on Physicochemical Properties of Gelatin Type A and Type B Methacryloyls. Macromol. Biosci. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and characterization of types A and B gelatin methacryloyl for bioink applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.; Weng Oh, S.K.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef]
- Ng, E.X.; Wang, M.; Neo, S.H.; Tee, C.A.; Chen, C.H.; Van Vliet, K.J. Dissolvable Gelatin-Based Microcarriers Generated through Droplet Microfluidics for Expansion and Culture of Mesenchymal Stromal Cells. Biotechnol. J. 2021, 16, 2000048. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Oh, J. Swelling characterization of photo-cross-linked gelatin methacrylate spherical microgels for bioencapsulation. e-Polymers 2014, 14, 161–168. [Google Scholar] [CrossRef]
- Samanipour, R.; Wang, Z.; Ahmadi, A.; Kim, K. Experimental and computational study of microfluidic flow-focusing generation of gelatin methacrylate hydrogel droplets. J. Appl. Polym. Sci. 2016, 133, 43701. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, C.; Min, Z.; Cai, W.; Zhang, X.; Li, W.; Zhang, A. Microfluidic Fabrication of Gelatin Acrylamide Microgels through Visible Light Photopolymerization for Cell Encapsulation. ACS Appl. Bio Mater. 2023, 6, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Habermann-Rottinghaus, S.M.; Murphy, K.P. Urea effects on protein stability: Hydrogen bonding and the hydrophobic effect. Proteins Struct. Funct. Genet. 1998, 31, 107–115. [Google Scholar] [CrossRef]
- Stumpe, M.C.; Grubmüller, H. Interaction of urea with amino acids: Implications for urea-induced protein denaturation. J. Am. Chem. Soc. 2007, 129, 16126–16131. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mukhopadhyay, C. Urea-mediated protein denaturation: A consensus view. J. Phys. Chem. B 2009, 113, 12816–12824. [Google Scholar] [CrossRef]
- Leuenberger, B.H. Investigation of viscosity and gelation properties of different mammalian and fish gelatins. Top. Catal. 1991, 5, 353–361. [Google Scholar] [CrossRef]
- Van Den Bosch, E.; Gielens, C. Gelatin degradation at elevated temperature. Int. J. Biol. Macromol. 2003, 32, 129–138. [Google Scholar] [CrossRef]
- Münstedt, H.; Schwarzl, F.R. Deformation and Flow of Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Osswald, T.; Rudolph, N. Polymer Rheology; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2014; pp. 101–141. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Wade, R.J.; Burdick, J.A. Engineering ECM signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef]
- Duconseille, A.; Andueza, D.; Picard, F.; Santé-Lhoutellier, V.; Astruc, T. Variability in pig skin gelatin properties related to production site: A near infrared and fluorescence spectroscopy study. Food Hydrocoll. 2017, 63, 108–119. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Olijve, J.; Mori, F.; Toda, Y. Influence of the molecular-weight distribution of gelatin on emulsion stability. J. Colloid Interface Sci. 2001, 243, 476–482. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Tadros, T.F. Volume 1 Interfacial Phenomena and Colloid Stability, Basic Principles; De Gruyter: Berlin, Germany, 2015. [Google Scholar] [CrossRef]
- Tadros, T.F. Volume 2 Interfacial Phenomena and Colloid Stability, Industrial Applications; De Gruyter: Berlin, Germany, 2015. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.; Malda, J.; Melchels, F.P. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Zhang, Q.; Piao, Y.; Bei, H.P.; Zhao, X. Biomimetic, Stiff, and Adhesive Periosteum with Osteogenic–Angiogenic Coupling Effect for Bone Regeneration. Small 2021, 17, 2006598. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Leister, N.; Yan, C.; Karbstein, H.P. Oil Droplet Coalescence in W/O/W Double Emulsions Examined in Models from Micrometer-to Millimeter-Sized Droplets. Colloids Interfaces 2022, 6, 12. [Google Scholar] [CrossRef]
- Halonen, S.; Kangas, T.; Haataja, M.; Lassi, U. Urea-Water-Solution Properties: Density, Viscosity, and Surface Tension in an Under-Saturated Solution. Emiss. Control Sci. Technol. 2017, 3, 161–170. [Google Scholar] [CrossRef]
- Huang, J.; Fu, D.; Wu, X.; Li, Y.; Zheng, B.; Liu, Z.; Zhou, Y.; Gan, Y.; Miao, Y.; Hu, Z. One-step generation of core–shell biomimetic microspheres encapsulating double-layer cells using microfluidics for hair regeneration. Biofabrication 2023, 15, 025007. [Google Scholar] [CrossRef]
- Guerrero, J.; Chang, Y.W.; Fragkopoulos, A.A.; Fernandez-Nieves, A. Capillary-Based Microfluidics—Coflow, Flow-Focusing, Electro-Coflow, Drops, Jets, and Instabilities. Small 2020, 16, 1904344. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef]
- Basu, A.S. Droplet morphometry and velocimetry (DMV): A video processing software for time-resolved, label-free tracking of droplet parameters. Lab Chip 2013, 13, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Noorani, B.; Xu, C. Effects of Encapsulated Cells on the Physical–Mechanical Properties and Microstructure of Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2019, 20, 5061. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.W. Effects of gelatin methacrylate bio-ink concentration on mechano-physical properties and human dermal fibroblast behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Rička, J.; Tanaka, T. Swelling of Ionic Gels: Quantitative Performance of the Donnan Theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.; Koenderink, G.H. A guide to mechanobiology: Where biology and physics meet. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 3043–3052. [Google Scholar] [CrossRef]
- Bandulasena, M.V.; Vladisavljević, G.T.; Benyahia, B. Versatile reconfigurable glass capillary microfluidic devices with Lego® inspired blocks for drop generation and micromixing. J. Colloid Interface Sci. 2019, 542, 23–32. [Google Scholar] [CrossRef]








| Product Number | Batch Number | Source | Bloom Strength | Nomenclature |
|---|---|---|---|---|
| G6144 | SLCH4483 | porcine | 80–120 g | p80 |
| G2625 | SLCC4273 | porcine | 175 g | p175 |
| G1890 | SLCC7838 | porcine | 300 g | p300 I |
| G1890 | SLBX2973 | porcine | 300 g | p300 II |
| 39465 | BCBW7164 | porcine | ultrahigh | pUH |
| G7765 | 038K0681 | fish | – | f |
| G6650 | SLCM1231 | bovine | 50–120 g | b50 |
| G9382 | SLCF9893 | bovine | 225 g | b225 |
| GelMA Sample | Concentration % (w/v) | Feed Rates mL min−1 | Feed Ratios x |
|---|---|---|---|
| f-100 MA | 15 | 12, 24, 60 | 10, 5, 2 |
| f-100 MA | 20 | 12, 24, 60 | 10, 5, 2 |
| p300 I-100 MA | 10 | 12, 24, 60 | 10, 5, 2 |
| p300 I-100 MA | 15 | 12, 24, 60 | 10, 5, 2 |
| p300 I-100 MA | 20 | 8, 24, 60 | 15, 10, 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalva Garces, D.; Appoldt, L.J.; Egner, J.; Leister, N.; Hubbuch, J. The Effect of Gelatin Source on the Synthesis of Gelatin-Methacryloyl and the Production of Hydrogel Microparticles. Gels 2023, 9, 927. https://doi.org/10.3390/gels9120927
Grijalva Garces D, Appoldt LJ, Egner J, Leister N, Hubbuch J. The Effect of Gelatin Source on the Synthesis of Gelatin-Methacryloyl and the Production of Hydrogel Microparticles. Gels. 2023; 9(12):927. https://doi.org/10.3390/gels9120927
Chicago/Turabian StyleGrijalva Garces, David, Luise Josephine Appoldt, Jasmin Egner, Nico Leister, and Jürgen Hubbuch. 2023. "The Effect of Gelatin Source on the Synthesis of Gelatin-Methacryloyl and the Production of Hydrogel Microparticles" Gels 9, no. 12: 927. https://doi.org/10.3390/gels9120927