Multicomponent Peptide-Based Hydrogels Containing Chemical Functional Groups as Innovative Platforms for Biotechnological Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Peptide Building Blocks
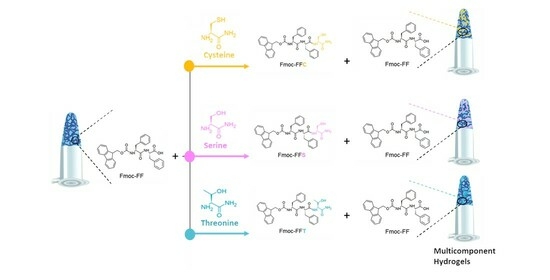
2.2. Hydrogel Formulation
2.3. Secondary Structural Characterization
2.4. SEM Characterization
2.5. Rheological Characterization
2.6. Cytotoxicity and Cell Adhesion Assays
3. Conclusions
4. Materials and Methods
4.1. Experiment
4.2. Solid Phase Peptide Synthesis
4.3. Peptide Characterization via HNMR Spectroscopy
4.3.1. Fmoc-FFC
4.3.2. Fmoc-FFS
4.3.3. Fmoc-FFT
4.4. Hydrogels Formulation
4.5. Hydrogel Swelling Studies
4.6. Circular Dichroism (CD) Studies
4.7. Fourier Transform Infrared (FT-IR) Spectroscopy
4.8. Thioflavin T (ThT) Spectroscopic Assay
4.9. Birefringence Congo Red (CR) Assay
4.10. Rheological Studies
4.11. Scanning Electron Microscopy (SEM)
4.12. Cell Lines
4.13. Cell Viability and Survival Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priya, S.; Batra, U.; Samshritha, R.N.; Sharma, S.; Chaurasiya, A.; Singhvi, G. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: A review. Int. J. Biol. Macromol. 2022, 218, 209–224. [Google Scholar] [CrossRef]
- Haidar, M.K.; Eroglu, H. Nanofibers: New Insights for Drug Delivery and Tissue Engineering. Curr. Top. Med. Chem. 2017, 17, 1564–1579. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkara, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Cox, H.; Mansfield, E.D.H.; Ellacott, S.H.; Peltier, R.; Brendel, J.C.; Hartlieb, M.; Waigh, T.A.; Perrier, S. Dual self-assembly of supramolecular peptide nanotubes to provide stabilisation in water. Nat. Commun. 2019, 10, 4708. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Zhang, J.; Li, J.; Liu, J.; Guan, S. Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA. Int. J. Mol. Sci. 2022, 23, 12071. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Balasco, N.; Sibillano, T.; Roviello, V.; Giannini, C.; Vitaliano, L.; Morelli, G.; Accardo, A. Fabrication of fluorescent nanospheres by heating PEGylated tetratyrosine nanofibers. Sci. Rep. 2021, 11, 2470. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Webber, M.; Appel, E.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nature Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, B.S.; Priyanka, G.; Rajalakshmi, S.; Sankar, R.; Sabreen, T.; Ravindran, J. Hydrogels: Potential aid in tissue engineering-a review. Polym. Bull. 2022, 79, 7009–7039. [Google Scholar] [CrossRef]
- Pentlavalli, S.; Coulter, S.; Laverty, G. Peptide Nanomaterials for Drug Delivery Applications. Curr. Protein Pept. Sci. 2020, 21, 401–412. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Sunwoo, S.H.; Kang, D.; Kang, T.; Cho, K.W.; Kim, M.; Park, O.K.; Jung, D.; Lee, J.; et al. Multifunctional injectable hydrogel for in vivo diagnostic and therapeutic applications. ACS Nano 2022, 16, 554–567. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Chan, S.Y.; Du, Z.; Yan, Y.; Wang, T.; Li, P.; Huang, W. Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. npj Flex Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Morelli, G.; Accardo, A. Self-Supporting Hydrogels Based on Fmoc-Derivatized Cationic Hexapeptides for Potential Biomedical Applications. Biomedicines 2021, 9, 678. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Inside Front Cover: Rigid, Self-Assembled Hydrogel Composed of a Modified Aromatic Dipeptide. Adv. Mater. 2006, 18, 136. [Google Scholar]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-Diphenylalanine self assembles to a hydrogel via a novel architecture based on π–π interlocked β-Sheets. Adv. Mater. 2008, 20, 37. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef]
- MacPherson, D.; Bram, Y.; Park, J.; Schwartz, R.E. Peptide-based scaffolds for the culture and maintenance of primary human hepatocytes. Sci. Rep. 2021, 11, 6772. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine as a suitable building block for the preparation of hybrid materials and their potential applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2019, 15, 487–496. [Google Scholar] [CrossRef]
- Rosa, E.; Gallo, E.; Sibillano, T.; Giannini, C.; Rizzuti, S.; Gianolio, E.; Scognamiglio, L.; Morelli, G.; Accardo, A.; Diaferia, C. Incorporation of PEG Diacrylates (PEGDA) Generates Hybrid Fmoc-FF Hydrogel Matrices. Gels 2022, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Hoque, J.; Varghese, S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019, 93, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Ding, Y.F.; Shui, M.; Yue, L.; Gao, C.; Wyman, I.W.; Wang, R. Supramolecular biomaterials for bio-imaging and imaging-guided therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, J.; Zhang, X.; Jiang, K.; Fan, H.; Li, Y.; Zhao, Y.; Liu, S.; Hao, D.; Li, G. Hydroxyapatite-Tethered Peptide Hydrogel Promotes Osteogenesis. Gels 2022, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Park, J.; Kim, S.; Cho, H.; Kim, H.J.; Park, S.; Shin, D.S. Tailored hydrogels for biosensor applications. J. Ind. Eng. Chem. 2020, 89, 1–12. [Google Scholar] [CrossRef]
- Wagner, H.J.; Mohsenin, H.; Weber, W. Synthetic Biology-Empowered Hydrogels for Medical Diagnostics. Adv. Biochem. Eng. Biotechnol. 2021, 178, 197–226. [Google Scholar]
- Misra, R.; Acharya, S. Smart nanotheranostic hydrogels for on-demand cancer management. Drug Discov. Today. 2021, 26, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Ambasht, P.K. Use of Group-Specific Reagents in Active Site Functional Group Elucidation I: Cys, Ser, Tyr, and Trp Residues. In Frontiers in Protein Structure, Function, and Dynamics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 71–94. [Google Scholar]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yuan, C.; Chakraborty, P.; Gilead, S.; Yan, X.; Gazit, E. Stoichiometry-controlled secondary structure transition of amyloid-derived supramolecular dipeptide co-assemblies. Chem. Commun. 2019, 2, 65. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, O.; Zhang, Y.; Adler-Abramovich, L.; Fan, X.; Mei, D.; Gazit, E.; Tao, K. Fmoc-diphenylalanine gelating nanoarchitectonics: A simplistic peptide self-assembly to meet complex applications. J. Colloid Interface Sci. 2023, 636, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Roy, S.; Javid, N.; Duncan, K.; Aitken, L.; Ulijn, R.V. Pathway-dependent gold nanoparticle formation by biocatalytic self-assembly. Nanoscale 2017, 9, 12330–12334. [Google Scholar] [CrossRef]
- Ryan, K.; Beirne, J.; Redmond, G.; Kilpatrick, J.I.; Guyonnet, J.; Buchete, N.V.; Kholkin, A.L.; Rodriguez, B.J. Nanoscale Piezoelectric Properties of Self-Assembled Fmoc-FF Peptide Fibrous Networks. ACS Appl. Mater. Interfaces 2015, 7, 12702–12707. [Google Scholar] [CrossRef]
- Hauser, K. Infrared Spectroscopy of Protein Folding, Misfolding and Aggregation. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1089–1095. [Google Scholar]
- Diaferia, C.; Balasco, N.; Altamura, D.; Sibillano, T.; Gallo, E.; Roviello, V.; Giannini, C.; Morelli, G.; Vitagliano, L.; Accardo, A. Assembly modes of hexaphenylalanine variants as function of the charge states of their terminal ends. Soft Matter 2018, 14, 8219–8230. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Espargaró, A.; Llabrés, S.; Saupe, S.J.; Curutchet, C.; Luque, F.J.; Sabaté, R. On the Binding of Congo Red to Amyloid Fibrils. Angew Chem. Int. Ed. Engl. 2020, 59, 8104–8107. [Google Scholar] [CrossRef]
- Wolfe, L.S.; Calabrese, M.F.; Nath, A.; Blaho, D.V.; Miranker, A.D.; Xiong, Y. Protein-induced photophysical changes to the amyloid indicator dye thioflavin T. Proc. Natl. Acad. Sci. USA 2010, 107, 16863–16868. [Google Scholar] [CrossRef]
- McFetridge, M.L.; Kulkarni, K.; Hilsenstein, V.; Del Borgo, M.P.; Aguilar, M.-I.; Ricardo, S.D. A comparison of fixation methods for SEM analysis of self-assembling peptide hydrogel nanoarchitecture. Nanoscale 2023, 15, 1431–1440. [Google Scholar] [CrossRef]
- Rosa, E.; Diaferia, C.; Gianolio, E.; Sibillano, T.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Accardo, A. Multicomponent Hydrogel Matrices of Fmoc-FF and Cationic Peptides for Application in Tissue Engineering. Macromol. Biosci. 2022, 22, 2200128. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Rosa, E.; Gallo, E.; Diaferia, C.; Morelli, G.; Stornaiuolo, M.; Accardo, A. Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Coin, I.; Beyermann, M.; Bienert, M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007, 2, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]








| Peptide | Formula | MWcalc. (a.m.u.) | MWdeter. (a.m.u.) | tR (min) |
|---|---|---|---|---|
| Fmoc-FFC | C36H36N4O5S | 636.7 | 636.3 | 21.5 |
| Fmoc-FFS | C36H36N4O6 | 620.6 | 621.4 | 19.8 |
| Fmoc-FFT | C37H38N4O6 | 634.7 | 635.4 | 20.3 |
| System | Ratio (w/w) | Swelling (%) | G′ (kPa) | G″ (kPa) | Tanδ | Gelation Time (min) |
|---|---|---|---|---|---|---|
| Fmoc-FFC/Fmoc-FF | 1/5 | 38.6 | 13.6 | 1.0 | 13.6 | 75 |
| 1/10 | 36.2 | 18.7 | 1.7 | 10.9 | 4.0 | |
| 1/20 | 33.2 | 16.6 | 1.6 | 10 | 2.6 | |
| Fmoc-FFS/Fmoc-FF | 1/5 | 34.0 | 9.7 | 1.2 | 7.8 | 90 |
| 1/10 | 34.8 | 17.3 | 1.9 | 9.3 | 20 | |
| 1/20 | 36.0 | 13.4 | 1.7 | 7.8 | 6.5 | |
| Fmoc-FFT/Fmoc-FF | 1/5 | 37.0 | 21.5 | 1.2 | 17.8 | 72 |
| 1/10 | 37.9 | 21.5 | 1.6 | 13.6 | 48 | |
| 1/20 | 41.0 | 13.8 | 1.2 | 11.6 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, S.; Gallo, E.; Diaferia, C.; Rosa, E.; Carrese, B.; Borbone, N.; Scognamiglio, P.L.; Franzese, M.; Oliviero, G.; Accardo, A. Multicomponent Peptide-Based Hydrogels Containing Chemical Functional Groups as Innovative Platforms for Biotechnological Applications. Gels 2023, 9, 903. https://doi.org/10.3390/gels9110903
Giordano S, Gallo E, Diaferia C, Rosa E, Carrese B, Borbone N, Scognamiglio PL, Franzese M, Oliviero G, Accardo A. Multicomponent Peptide-Based Hydrogels Containing Chemical Functional Groups as Innovative Platforms for Biotechnological Applications. Gels. 2023; 9(11):903. https://doi.org/10.3390/gels9110903
Chicago/Turabian StyleGiordano, Sabrina, Enrico Gallo, Carlo Diaferia, Elisabetta Rosa, Barbara Carrese, Nicola Borbone, Pasqualina Liana Scognamiglio, Monica Franzese, Giorgia Oliviero, and Antonella Accardo. 2023. "Multicomponent Peptide-Based Hydrogels Containing Chemical Functional Groups as Innovative Platforms for Biotechnological Applications" Gels 9, no. 11: 903. https://doi.org/10.3390/gels9110903