(N-Alkyloxalamido)-Amino Acid Amides as the Superior Thixotropic Phase Selective Gelators of Petrol and Diesel Fuels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Gelation Properties
2.2. Phase Selective Gelation
2.3. TEM, SEM Microscopy and DSC Study
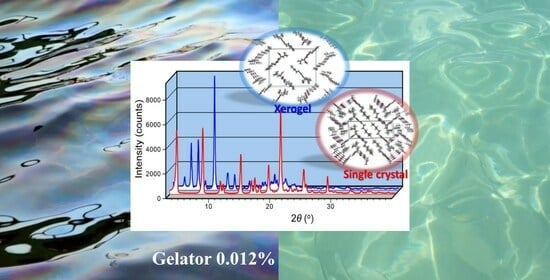
2.4. Crystal Structure and XRPD Studies
2.5. NMR and FTIR Study
2.6. Oscillatory Rheology of the Gels
2.6.1. Amplitude Sweep
2.6.2. Frequency Sweep
2.6.3. Thixotropic Properties
3. Conclusions
4. Materials and Methods
4.1. Determination of Gelling Properties
4.2. TEM Investigation
4.3. SEM Microscopy
4.4. NMR Spectroscopy
4.5. Differential Scanning Calorimetry
4.6. FTIR Measurements
4.7. Single Crystal Analysis
4.8. XRPD Studies
4.9. Structure Solution from XRPD Data
4.10. Rheological Investigation
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.D.; Chen, L.M.; Zhang, M.M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W. Anion-tuned supramolecular gels: A natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 3686–3699. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, J.L. Soft Fibrillar Materials: Fabrication and Applications; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]
- Sangeetha, M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Jung, J.H.; Shinkai, S. Gels as Templates for Nanotubes. Top. Curr. Chem. 2004, 248, 223–260. [Google Scholar]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.D.; Kuhbeck, D.; Koopmans, R.J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Vibhute, A.M.; Sureshan, K.M. How Far Are We in Combating Marine Oil Spills by Using Phase-Selective Organogelators? ChemSusChem 2020, 13, 5343–5360. [Google Scholar] [CrossRef]
- Bachl, J.; Oehm, S.; Mayr, J.; Cativiela, C.; Marrero-Tellado, J.J.; Díaz Díaz, D. Supramolecular Phase-Selective Gelation by Peptides Bearing Side-Chain Azobenzenes: Effect of Ultrasound and Potential for Dye Removal and Oil Spill Remediation. Int. J. Mol. Sci. 2015, 16, 11766–11784. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Krishnan-Ghosh, Y. First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Commun. 2001, 2, 185–186. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Konda, M.; Maity, I.; Rasale, D.B.; Das, A.K. A New Class of Phase-Selective Synthetic β-Amino Acid Based Peptide Gelator: From Mechanistic Aspects to Oil Spill Recovery. ChemPlusChem 2014, 79, 1482–1488. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. A mannitol based phase selective supergelator offers a simple, viable and greener method to combat marine oil spills. Chem. Commun. 2012, 48, 5250–5252. [Google Scholar] [CrossRef]
- Pelletier, É.; Siron, R. Silicone-based polymers as oil spill treatment agents. Environ. Toxicol. Chem. 1999, 18, 813–818. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Guterman, L. Conservation biology. Exxon Valdez turns 20. Science 2009, 323, 1558–1559. [Google Scholar] [CrossRef]
- Basak, S.; Nanda, J.; Banerjee, A. A new aromatic amino acid based organogel for oil spill recovery. J. Mater. Chem. 2012, 22, 11658–11664. [Google Scholar] [CrossRef]
- Monograph in Supramolecular Chemistry. Molecular Gels, Structure and Dynamics; Weiss, R.G., Ed.; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Guenet, J.M. Organogels: Thermodynamics, Structure, Solvent Role and Properties; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Terech, P.; Weiss, R.G. (Eds.) Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Feng, G.; Chen, H.; Cai, J.; Wen, J.; Liu, X. L-Phenylalanine based low-molecular-weight efficient organogelators and their selective gelation of oil from oil/water mixtures. Soft Mater 2014, 12, 403–410. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Vemula, P.K.; Kumar, R.; Raghavan, S.R.; John, G. Sugar-Derived Phase-Selective Molecular Gelators as Model Solidifiers for Oil Spills. Angew. Chem. Int. Ed. 2010, 49, 7695–7698. [Google Scholar] [CrossRef]
- Ballabh, A.; Trivedi, D.R.; Dastidar, P. New Series of Organogelators Derived from a Combinatorial Library of Primary Ammonium Monocarboxylate Salts. Chem. Mater. 2006, 18, 3795–3800. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Cho, J.-Y.; Smith, B.; Carlini, R.; Godbert, N. Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption. Gels 2022, 8, 285. [Google Scholar] [CrossRef]
- Mukherjee, S.; Shang, C.; Chen, X.; Chang, X.; Liu, K.; Yu, C.; Fang, Y. N-Acetylglucosamine-based efficient, phase-selective organogelators for oil spill remediation. Chem. Commun. 2014, 50, 13940–13943. [Google Scholar] [CrossRef]
- Srivastava, B.K.; Manheri, M.K. Aryl-triazolyl peptides for efficient phase selective gelation and easy removal of dyes from water. RSC Adv. 2016, 6, 29197–29201. [Google Scholar] [CrossRef]
- Ren, C.L.; Ng, G.H.B.; Wu, H.; Chan, K.-H.; Shen, J.; Teh, C.; Ying, J.Y.; Zeng, H.Q. Instant Room-Temperature Gelation of Crude Oil by Chiral Organogelators. Chem. Mater. 2016, 28, 4001–4008. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Handore, K.; Sureshan, K.M. Soft Optical Devices from Self-Healing Gels Formed by Oil and Sugar-Based Organogelators. Angew. Chem. Int. Ed. 2011, 50, 8021–8024. [Google Scholar] [CrossRef]
- Peng, J.; Liu, K.; Liu, X.; Xia, H.; Liu, J.; Fang, Y. New dicholesteryl-based gelators: Gelling ability and selective gelation of organic solvents from their mixtures with water at room temperature. New J. Chem. 2008, 32, 2218–2224. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Venkatanarayana, M.; Sureshan, K.M. A Sugar-Based Gelator for Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2016, 55, 7782–7785. [Google Scholar] [CrossRef]
- Ren, C.L.; Shen, J.; Chen, F.; Zeng, H.Q. Rapid Room-Temperature Gelation of Crude Oils by a Wetted Powder Gelator. Angew. Chem. Int. Ed. 2017, 56, 3847–3851. [Google Scholar] [CrossRef]
- Pathak, N.P.; Rajkamal; Yadav, S. A gelator–starch blend for dry powder based instant solidification of crude oil at room temperature. Chem. Commun. 2020, 56, 2999–3002. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. Organogelator–Cellulose Composite for Practical and Eco-Friendly Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2017, 56, 9405–9409. [Google Scholar] [CrossRef] [PubMed]
- Frkanec, L.; Žinić, M. Chiral bis(amino acid)- and bis(amino alcohol)-oxalamidegelators. Gelation properties, self-assembly motifs and chirality effects. Chem. Commun. 2010, 46, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Makarević, J.; Jokić, M.; Perić, B.; Tomišić, V.; Kojić-Prodić, B.; Žinić, M. Bis(Amino Acid) Oxalyl Amides as Ambidextrous Gelators of Water and Organic Solvents: Supramolecular Gels with Temperature Dependent Assembly/Dissolution Equilibrium. Chem. Eur. J. 2001, 7, 3328–3341. [Google Scholar] [CrossRef]
- Vujičić, N.Š.; Glasovac, Z.; Zweep, N.; van Esch, J.; Vinković, M.; Popović, J.; Žinić, M. Chiral hexa- and nona-methylene bridged bis(L-Leu-oxalamide) gelators. First oxalamide gels containing aggregates with chiral morphology. Chem. A Eur. J. 2013, 19, 8558–8572. [Google Scholar] [CrossRef]
- Azyat, K.; Makeiff, D.; Smith, B.; Wiebe, M.; Launspach, S.; Wagner, A.; Kulka, M.; Godbert, N. The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides. Gels 2023, 9, 5. [Google Scholar] [CrossRef]
- Zanna, N.; Tomasini, C. Peptide-based physical gels endowed with thixotropic behaviour. Gels 2017, 3, 39. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Mallia, A.; Weiss, R. Correlations between thixotropic and structural properties of molecular gels with crystalline networks. Soft Matter 2016, 12, 3665–3676. [Google Scholar] [CrossRef]
- Chen, S.; Fan, Y.; Song, J.; Xue, B. The remarkable role of hydrogen bond, halogen, and solvent effect on self-healing supramolecular gel. Mater. Today Chem. 2022, 23, 100719. [Google Scholar] [CrossRef]
- Zhang, Y.; Weiss, R.G. How do H-bonding interactions control viscoelasticity and thixotropy of molecular gels? Insights from mono-, di- and tri-hydroxymethylated alkanamide gelators. J. Colloid Interface Sci. 2017, 486, 359–371. [Google Scholar] [CrossRef]
- Žinić, M.; Makarević, J. Amphiphilic Oxalamide Organogelators Designed for Gelation of Organic Solvents, Water and Hydrocarbon Commercial Fuels. U.S. Patent No. 8,637,705 B2, 28 January 2014. [Google Scholar]
- Makarević, J.; Jokić, M.; Raza, Z.; Štefanić, Z.; Kojić-Prodić, B.; Žinić, M. Chiral bis (amino alcohol) oxalamide gelators—Gelation properties and supramolecular organization: Racemate versus pure enantiomer gelation. Chem. Eur. J. 2003, 9, 5567–5580. [Google Scholar] [CrossRef] [PubMed]
- Čaplar, V.; Frkanec, L.; Šijaković Vujičić, N.; Žinić, M. Positionally Isomeric Organic Gelators: Structure–Gelation Study, Racemic versus Enantiomeric Gelators, and Solvation Effects. Chem. Eur. J. 2010, 16, 3066–3082. [Google Scholar] [CrossRef] [PubMed]
- Džolić, Z.; Wolsperger, K.; Žinić, M. Synergic effect in gelation by two-component mixture of chiral gelators. New J. Chem. 2006, 30, 1411–1419. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, A.; Ye, L.; Feng, Z. Organo- and hydrogels derived from cyclo(L-Tyr-L-Lys) and its ε-amino derivatives. Soft Matter 2009, 5, 1474–1482. [Google Scholar] [CrossRef]
- Escuder, B.; Llusar, M.; Miravet, J.F. Insight on the NMR study of supramolecular gels and its application to monitor molecular recognition on self-assembled fibers. J. Org. Chem. 2006, 71, 7747–7752. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Coates, I.A.; Bouche-teau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-molecular-weight gelators: Elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Jonkheijm, P.; van der Shoot, P.; Schenning, A.P.H.J.; Meijer, E.W. Probing the solvent-assisted nucleation pathway in chemical self-assembly. Science 2006, 313, 80–83. [Google Scholar] [CrossRef]
- Cantekin, S.; Nakano, Y.; Everts, J.C.; van der Schoot, P.; Meijer, E.W.; Palmans, A.R.A. A stereoselectively deuterated supramolecular motif to probe the role of solvent during self-assembly processes. Chem. Commun. 2012, 48, 3803–3805. [Google Scholar] [CrossRef]
- Pinault, T.; Isare, B.; Boutellier, L. Solvents with Similar Bulk Properties Induce Distinct Supramolecular Architectures. ChemPhysChem 2006, 7, 816–819. [Google Scholar] [CrossRef]
- Mukai, M.; Minamikawa, H.; Aoyagi, M.; Asakawa, M.; Shimizu, T.; Kogiso, M. Solvent-chirality selective organogelation by chiral aspartame lipids. Soft Matter 2012, 8, 11979–11981. [Google Scholar] [CrossRef]
- Niu, L.; Song, J.; Li, J.; Tao, N.; Lua, M.; Fan, K. Solvent effects on the gelation performance of melamine and 2-ethylhexylphosphoric acid mono-2-ethylhexyl ester in water–organic mixtures. Soft Matter 2013, 9, 7780–7786. [Google Scholar] [CrossRef]
- Zhu, G.; Dordick, J.S. Solvent Effect on Organogel Formation by Low Molecular Weight Molecules. Chem. Mater. 2006, 18, 5988–5995. [Google Scholar] [CrossRef]
- Bouguet-Bounnet, S.; Yemloul, M.; Canet, D. New Application of Proton Nuclear Spin Relaxation Unraveling the Intermolecular Structural Features of Low-Molecular-Weight Organogel Fibers. J. Am. Chem. Soc. 2012, 134, 10621–10627. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.R.; Ballabh, A.; Dastidar, P.; Ganguly, B. Structure–Property Correlation of a New Family of Organogelators Based on Organic Salts and Their Selective Gelation of Oil from Oil/Water Mixtures. Chem. Eur. J. 2004, 10, 5311–5322. [Google Scholar] [CrossRef]
- Agilent Technologies. Xcalibur/SuperNova CCD System, CrysAlisPro Software System, Version 71.37.35; Agilent Technologies UK Ltd.: Oxford, UK, 2013. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta. Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Altomare, A.; Camalli, M.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R. Advances in powder diffraction pattern indexing: N-TREOR09. J. Appl. Cryst. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- de Wolff, P.M. A simplified criterion for the reliability of a powder pattern indexing. J. Appl. Crystallogr. 1968, 1, 108–113. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab Initio Structure Determination of LiSbWO6 by X ray Powder Diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]











| V/mL | ||||
|---|---|---|---|---|
| 9 | 10 | 11 | 12 | |
| petrol | 45.80 | 79.20 | 7.00 | 10.30 |
| diesel | 36.10 | 67.20 | 10.0 | 42.40 |
| water | 6.85 | NG | NS | NG |
| DMSO | NG | NG | NG | NG |
| DMSO/water | 1.5 + 0.75 | 2.15 + 1.65 | 7.50 + 20.50 | 17.10 + 20.70 |
| DMF/water | 0.6 + 0.5 | 1.15 + 2.55 | 3.45 + 3.45 | 10.35 + 13.30 |
| EtOH | 0.25 | NG | NG | 1.05 |
| THF | 0.1 | NG | 0.50 | 0.15 |
| EtOAc | 0.2 | NG | 1.00 | NG |
| acetone | NG | NG | NG | NG |
| CH2Cl2 | 1.05 | 750 | NS | 750 |
| CH2Cl2/DMSO | 0.40 + 0.04 | |||
| CH3CN | NG | NG | NG | 1.45 |
| toluene | 24.95 | 16.20 | 4.00 | 15.20 |
| p-xylene | 33.60 | 68.00 | 7.00 | 5.25 |
| decaline | 37.40 | 106.25 | 26.00 | 24.10 |
| tetraline | 0.5 | 0.15 | 0.50 | 0.30 |
| Yield Point/Pa | |||||
|---|---|---|---|---|---|
| Gelator 10 | G′/Pa | G′′/Pa | tanδ | τ/Pa | γ/% |
| petrol | 54,996.0 | 7002.1 | 0.12 | 828.5 | 3.24 |
| toluene | 41,623.3 | 5738.1 | 0.13 | 393.5 | 1.49 |
| diesel | 3105.5 | 443.3 | 0.14 | 18.87 | 0.78 |
| 3ITT Test | ||
|---|---|---|
| Sample 10 | Recovery (t = 30 s)/% | Recovery (t = 300 s)/% |
| petrol | 50.1 | 71.2 |
| toluene | 56.7 | 77.1 |
| diesel | 62.6 | 76.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujičić, N.Š.; Makarević, J.; Popović, J.; Štefanić, Z.; Žinić, M. (N-Alkyloxalamido)-Amino Acid Amides as the Superior Thixotropic Phase Selective Gelators of Petrol and Diesel Fuels. Gels 2023, 9, 852. https://doi.org/10.3390/gels9110852
Vujičić NŠ, Makarević J, Popović J, Štefanić Z, Žinić M. (N-Alkyloxalamido)-Amino Acid Amides as the Superior Thixotropic Phase Selective Gelators of Petrol and Diesel Fuels. Gels. 2023; 9(11):852. https://doi.org/10.3390/gels9110852
Chicago/Turabian StyleVujičić, Nataša Šijaković, Janja Makarević, Jasminka Popović, Zoran Štefanić, and Mladen Žinić. 2023. "(N-Alkyloxalamido)-Amino Acid Amides as the Superior Thixotropic Phase Selective Gelators of Petrol and Diesel Fuels" Gels 9, no. 11: 852. https://doi.org/10.3390/gels9110852