Biomedical Applications of Thermosensitive Hydrogels for Controlled/Modulated Piroxicam Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogel Polymerization
2.2. Residual Reactants Analysis
2.3. Differential Scanning Calorimetry (DSC) Analysis
2.4. Swelling Study
2.4.1. Swelling Reversibility
2.4.2. Analysis of the Hydrogels Swelling Kinetics
2.4.3. Determination of the Order of Reaction for Swelling Process
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5.1. FTIR Spectrum Analysis of Piroxicam
2.5.2. FTIR Spectrum Analysis of p(NiPAm-HPMet) with Incorporated Piroxicam
2.6. Piroxicam Incorporation Efficiency into p(NiPAm-HPMet) Hydrogels
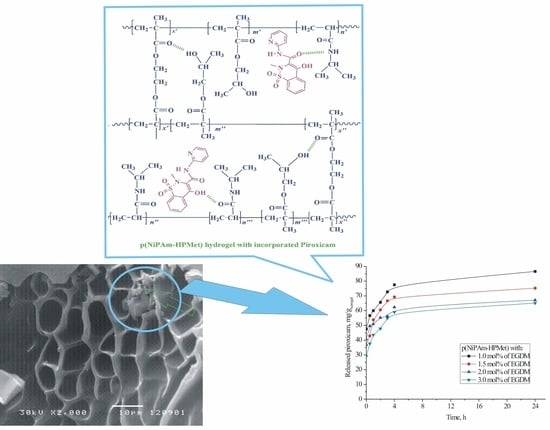
2.7. SEM Analysis of the Copolymer with Incorporated Piroxicam
2.8. In Vitro Thermoresponsive Piroxicam Release from p(NiPAm-HPMet) Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Thermosensitive Hydrogels Synthesis
4.3. Residual Reactants Analysis
4.4. Swelling Study
4.4.1. Kinetic
4.4.2. The Order of a Swelling Reaction
4.5. Incorporation of Piroxicam into the Thermosensitive Hydrogels
4.6. In Vitro Piroxicam Release from Thermosensitive Hydrogels
4.7. Characterization
4.7.1. DSC Method
4.7.2. FTIR Method
4.7.3. Lyophilization Process
4.7.4. Scanning Electron Microscope (SEM) Method
4.8. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, R.S.; Carrupt, P.A.; El Tayar, N.; Giroud, Y.; Andrade, P.; Testa, B. Physicochemical and structural properties of non-steroidal anti-inflammatory oxicams. Helv. Chim. Acta 1993, 76, 842–854. [Google Scholar] [CrossRef]
- Bordner, J.; Hammen, P.D.; Whipple, E.B. Deuterium isotope effects on carbon-13 NMR shifts and the tautomeric equilibrium in N-substitued pyridyl derivatives of Piroxicam. J. Am. Chem. Soc. 1989, 111, 6572–6578. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, D.W.; Kang, S.G.; Yoon, M.; Kim, D. Excited-state intramolecular proton transfer emission of Piroxicam in aqueous β-cyclodextrin solutions. J. Lumin. 1994, 59, 209–217. [Google Scholar] [CrossRef]
- Yoon, M.; Choi, H.N.; Kwon, H.W.; Park, K.H. Solvent dependence of absorption and fluorescence spectra of Piroxicam. A possible intramolecular proton transfer in the excited state. Bull Korean Chem. Soc. 1988, 9, 171–175. [Google Scholar] [CrossRef]
- Banerjee, R.; Chakraborty, H.; Sarkar, M. Photophysical studies of oxicam group of NSAIDs: Piroxicam, meloxicam and tenoxicam. Spectrochim. Acta A 2002, 59, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.M.; Costa, S.M.B. Hydrogen bonding effects in the photophysics of a drug. Piroxicam, in homogeneous media and dioxane–water mixtures. Phys. Chem. Chem. Phys. 1999, 1, 4213–4219. [Google Scholar] [CrossRef]
- Sahu, C.R. Mechanisms involved in toxicity of liver caused by piroxicam in mice and protective effects of leaf extract of Hibiscus rosa-sinensis L. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Shimizu, H.; Tokuyama, S.; Hariya, T.; Soh, I.; Sueki, H.; Kuroiwa, Y. Antigenic characterization in ampiroxicam-induced photosensitivity using an in vivo model of contact hypersensitivity. J. Dermatol. Sci. 1999, 21, 170–175. [Google Scholar] [CrossRef]
- Kurumaji, Y. Ampiroxicam-induced photosensitivity. Contact Derm. 1996, 34, 298. [Google Scholar] [CrossRef]
- Nikolić, V.D.; Ilić-Stojanović, S.S.; Nikolić, L.B.; Cakić, M.D.; Zdravković, A.S.; Kapor, A.J.; Popsavin, M.M. Photostability of piroxicam in the inclusion complex with 2-hydroxypropyl-β-cyclodextrin. Hem. Ind. 2014, 68, 107–116. [Google Scholar] [CrossRef]
- Hideyoshi, K.; Youichi, N.; Akira, I. Piroxicam tablets and production process thereof. EP Patent 0449167B1, 25 March 1991. [Google Scholar]
- Albertini, B.; Cavallari, C.; Passerini, N.; González-Rodríguez, M.L.; Rodrigueza, L. Evaluation of β-lactose, PVP K12 and PVP K90 as excipients to prepare piroxicam granules using two wet granulation techniques. J. Pharm. Biopharm. 2003, 56, 479–487. [Google Scholar] [CrossRef]
- Giunchedi, P.; Conte, U.; Chetoni, P.; Saettone, M. Pectin microspheres as ophthalmic carriers for piroxicam: Evaluation in vitro and in vivo in albino rabbits. Eur. J. Pharm. Sci. 1999, 9, 1–7. [Google Scholar] [CrossRef]
- Ravikiran, N.; Palanisamy, S.; Arunachalam, A.; Karthikeyan, M.; Kumar, A.S.; Santhi, P. Design and evaluation of Orodispersible tablet of Piroxicam using different Superdisintegrants. Int. J. Drug Dev. Res. 2010, 1, 349–374. [Google Scholar]
- Hansen, J.E.; Pines, E.; Fleming, G.R. Excited-state proton transfer of protonated 1-aminopyrene complexed with beta-cyclodextrin. J. Phys. Chem. B 1992, 96, 6904–6910. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Das, K.; Das, S.; Datta, A.; Nath, D.; Bhattacharyya, K. Excited-state intramolecular proton transfer of 2-(29-hydroxyphenyl)benzimidazole in micelles. J. Phys. Chem. 1995, 99, 17711–17714. [Google Scholar] [CrossRef]
- Osadebe, P.O.; Onugwu, L.E.; Attama, A.A. Energetics of the interaction between piroxicam and beta-cyclodextrin (β-CD) in inclusion complexes. Sci. Res. Essays 2008, 3, 086–093. [Google Scholar]
- Cavallari, C.; Albertini, B.; González-Rodrĺguez, M.L.; Rodriguez, L. Improved dissolution behaviour of steam-granulated piroxicam. Eur. J. Pharm. Biopharm. 2001, 54, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, T.; Piel, G.; Henry de Hassonville, S.; Evrard, B.; Delattre, L. Determination of the free / included piroxicam ratio in cyclodextrin complexes: Comparison between UV spectrophotometry and differential scanning calorimetry. Eur. J. Pharm. Sci. 2002, 15, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Lai, J.; Lu, Y.; Yin, Z.; Wu, W. Piroxicam/2-Hydroxypropyl-b-Cyclodextrin Inclusion Complex Prepared by a New Fluid-Bed Coating Technique. J. Pharm. Sci. 2009, 98, 665–675. [Google Scholar] [CrossRef]
- Negrin, C.M.; Delgado, A.; Llabres, M.; Evora, C. Methadone implants for methadone maintenance treatment. In vitro and in vivo animals’ studies. J. Control Release 2004, 95, 413–421. [Google Scholar] [CrossRef]
- Cabana, A.; Aitkadi, A.; Juhasz, J. Study of the gelation process of polyethylene oxidepolypropyleneoxidepolyethylene oxide copolymer (poloxamer 407) aqueous solution. J. Colloid Interface Sci. 1997, 190, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Hoffmann, H.; Ulbricht, W. The aggregation behavior of poly(oxyethylene)–poly(oxypropylene)– poly(oxyethylene)-block-copolymers in aqueous solution. Colloid Polym. Sci. 1990, 268, 101–117. [Google Scholar]
- Xuan, J.J.; Balakrishnan, B.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Verma, A.; Ram, V. Evaluation of chitosan-hydroxy propyl methyl cellulose as a single unit hydrodynamically balanced sustained release matrices for stomach specific delivery of Piroxicam. MOJ Bioequiv. Availab. 2016, 2, 00014. [Google Scholar]
- Rahmani Del Bakhshayesh, A.; Akbarzadeh, A.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Abedelahi, A. Preparation and characterization of novel anti-inflammatory biological agents based on piroxicam-loaded poly-ε-caprolactone nano-particles for sustained NSAID delivery. Drug Deliv. 2020, 27, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Oddo, L.; Masci, G.; Di Meo, C.; Capitani, D.; Mannina, L.; Lamanna, R.; De Santis, S.; Alhaique, F.; Coviello, T.; Matricardi, P. Novel thermosensitive calcium alginate microspheres: Physico-chemical characterization and delivery properties. Acta Biomater. 2010, 6, 3657–3664. [Google Scholar] [CrossRef]
- Qindeel, M.; Ahmed, N.; Sabir, F.; Khan, S.; Ur-Rehman, A. Development of novel pH-sensitive nanoparticles loaded hydrogel for transdermal drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 629–641. [Google Scholar] [CrossRef]
- Boonme, P.; Molee, N.; Kanchanaclod, T.; Yang, H. Formulation and characterization of piroxicam-loaded water-in-oil microemulsions. Prog. Appl. Sci. Technol. 2020, 10, 43–49. [Google Scholar]
- Park, C.W.; Ma, K.W.; Jang, S.W.; Son, M.; Kang, M.J. Comparison of piroxicam pharmacokinetics and anti-inflammatory effect in rats after intra-articular and intramuscular administration. Biomol. Ther. 2014, 22, 260. [Google Scholar] [CrossRef] [Green Version]
- Nizam El-Din, H.M. Characterization and caffeine release properties of N-isopropylacrylamide/hydroxypropyl methacrylate, copolymer hydrogel synthesized by gamma radiation. J. Appl. Polym. Sci. 2011, 119, 577–585. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Milić, J.; Stamenković, J.; Nikolić, G.M.; Petrović, S.D.; Kapor, A. Potential application of thermosensitive hydrogels for controlled release of phenacetin. Chem. Ind. 2012, 66, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Ilić-Stojanović, S.S.; Nikolić, L.B.; Nikolić, V.D.; Milić, J.R.; Stamenković, J.; Nikolić, G.M.; Petrović, S.D. Synthesis and characterization of thermosensitive hydrogels and the investigation of modified release of ibuprofen. Hem. Ind. 2013, 67, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Petrović, S.; Oro, V.; Mitić, Ž.; Najman, S. Semi-Crystalline copolymer hydrogels as smart drug carriers: In vitro thermo-responsive naproxen release study. Pharmaceutics 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.; Zdravković, A.; Nikolić, V.D. Synthesis and characterization of poly (N-isopropylmethacrylamide-co-N-isopropylacrylamide) copolymers. Hem. Ind. 2020, 74, 103–117. [Google Scholar] [CrossRef]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.S.; Nikolić, V.D.; Petrović, S.M.; Zdravković, A.S. Hydrogels based on N-isopropylmethacrylamide and N-isopropylacrylamide. Adv. Technol. 2018, 7, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Kostic, M.; Pejcic, A.; Igic, M.; Gligorijevic, N. Adverse reactions to denture resin materials. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5298–5305. [Google Scholar]
- Ilić-Stojanović, S. Synthesis and Characterization of Negatively Thermosensitive Hydrogels; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2015; ISBN 978-3-659-47484-2. [Google Scholar]
- Al-Obaidi, H.; Buckton, G. Evaluation of griseofulvin binary and ternary solid dispersions with HPMCAS. AAPS Pharm. SciTechnol. 2009, 10, 1172–1177. [Google Scholar] [CrossRef] [Green Version]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Budinski-Simendić, J.; Kapor, A.; Nikolić, G.M. The structure characterization of thermosensitive poly(N-isopropylacrylamide-co-2-hydroxypropyl methacrylate) hydrogel. Polym. Int. 2013, 63, 973–981. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.S.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Li, H. Smart Hydrogel Modeling; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK.; New York, NY, USA, 2009. [Google Scholar]
- Cai, W.; Anderson, E.C.; Gupta, R.B. Separation of lignin from aqueous mixtures by ionic and nonionic temperature-sensitive hydrogels. Ind. Eng. Chem. Res. 2001, 40, 2283–2288. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Johannes, I.; Hunt, C.A.; Firestone, B.A. Buffer effects on swelling kinetics in polybasic gels. Pharm. Res. 1992, 19929, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A. Responsive Gels: Volume Transitions. In Advanced in Polymer Science; Dusek, I.K., Ed.; 109; Springer: Berlin/Heidelberg, Germany, 1993; pp. 233–267. [Google Scholar]
- Andersson, M.; Axelsson, A.; Zacchi, G. Diffusion of glucose and insulin in NiPAAmgel. Int. J. Pharmacol. 1997, 157, 199–208. [Google Scholar] [CrossRef]
- Andersson, M.; Axelsson, A.; Zacchi, G. Swelling kinetics of poly(N-isopropylacrylamide) gel. J. Control Release 1998, 50, 273–281. [Google Scholar] [CrossRef]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Control Release 2004, 98, 97–114. [Google Scholar] [CrossRef]
- Jovašević, J.S.; Mićić, M.M.; Suljovrujić, E.H.; Filipović, J.M.; Dimitrijević, S.I.; Tomić, S.L. Antimicrobial activity of hybrid hydrogels based on poly(vinylpyrrolidone) containing silver. Hem. Ind. 2010, 64, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Mihalic, M.; Hofman, H.; Kuftinec, J.; Krile, B.; Caplar, V.; Kajfez, F.; Blazevic, M.P. Analytical Profiles of Drug Substances 15; Florey, K., Ed.; Academic Press: New York, NY, USA, 1986; pp. 509–531. [Google Scholar]
- Zhang, X.Z.; Zhuo, R.X. Preparation of fast responsive, thermally sensitive poly(N-isopropylacrylamide) gel. Eur. Polym. J. 2000, 36, 2301–2303. [Google Scholar] [CrossRef]
- Piringer, O.G.; Baner, A.L. (Eds.) Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 135. [Google Scholar]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.B.; Nikolić, V.; Stanković, M.; Stamenković, J.; Mladenović-Ranisavljević, I.; Petrović, S. Influence of monomer and crosslinker molar ratio on the swelling behaviour of thermosensitive hydrogels. Chem. Ind. Chem. Eng. 2012, 18, 1–9. [Google Scholar] [CrossRef]







| p(NiPAm-HPMet) with mol% of Crosslinker EGDM | NiPAm, % | HPMet, % | EGDM, % |
|---|---|---|---|
| 1.0 | 0.36 | 0.27 | 0.66 |
| 1.5 | 0.45 | 0.32 | 0.62 |
| 2.0 | 0.46 | 0.47 | 0.45 |
| 3.0 | 0.47 | 0.38 | 0.31 |
| p(NiPAm-HPMet) with mol% of Crosslinker EGDM | Tg1, ( °C) | Tg2, ( °C) | Tg3, ( °C) | Tm1, ( °C) | Tm2, ( °C) | ΔHm, (J∙g−1) |
|---|---|---|---|---|---|---|
| 1.0 | 71.26 | - | 129.81 | 152.36 | 156.49 | 6.48 |
| 2.0 | 63.40 | 81.84 | 134.67 | 150.93 | 155.27 | 3.55 |
| 3.0 | 62.88 | 81.05 | 135.95 | 150.72 | 153.14 | 2.94 |
| p(NiPAm-HPMet) with mol% of EGDM | αe | n | k × 102 (min–1/2) | R2 | D (cm2/min) |
|---|---|---|---|---|---|
| 1.0 | 26.70 | 0.395 | 6.323 | 0.997 | 1.041 × 10−5 |
| 1.5 | 18.04 | 0.575 | 2.987 | 0.996 | 8.872 × 10−7 |
| 2.0 | 15.10 | 0.500 | 2.720 | 0.989 | 1.414 × 10−6 |
| 3.0 | 12.03 | 0.435 | 2.472 | 0.996 | 0.186 × 10−6 |
| p(NiPAm-HPMet) with mol% of EGDM | αe | n | k × 102 (min–1/2) | R2 | D × 104 (cm2/min) |
|---|---|---|---|---|---|
| 1.0 | 3.94 | 0.434 | 0.095 | 0.999 | 2.979 |
| 1.5 | 2.61 | 0.433 | 0.747 | 0.995 | 1.206 |
| 2.0 | 2.04 | 0.290 | 0.142 | 0.997 | 2.016 |
| 3.0 | 1.53 | 0.379 | 0.111 | 0.983 | 2.594 |
| p(NiPAm-HPMet) with mol% of EGDM | αe (exp) | αe (I order) | K·103, min−1 | R2 | αe (II order) | K·103, min−1 | R2 |
|---|---|---|---|---|---|---|---|
| 1.0 | 26.70 | 28.621 | 4.54 | 0.968 | 27.504 | 0.344 | 0.999 |
| 1.5 | 18.04 | 19.628 | 3.87 | 0.974 | 18.693 | 1.014 | 0.999 |
| 2.0 | 15.10 | 16.002 | 2.71 | 0.988 | 15.344 | 3.118 | 0.999 |
| 3.0 | 12.03 | 12.914 | 3.54 | 0.990 | 12.413 | 2.247 | 0.999 |
| p(NiPAm-HPMet) with mol% of EGDM | αe (exp) | αe (I order) | K·103, min−1 | R2 | αe (II order) | K·103, min−1 | R2 |
|---|---|---|---|---|---|---|---|
| 1.0 | 3.94 | 4.672 | 7.58 | 0.994 | 4.223 | 6.081 | 0.999 |
| 1.5 | 2.61 | 3.254 | 7.24 | 0.989 | 2.978 | 5.173 | 0.999 |
| 2.0 | 2.04 | 2.904 | 6.47 | 0.988 | 2.561 | 5.417 | 0.999 |
| 3.0 | 1.53 | 1.771 | 6.89 | 0.991 | 1.65 | 4.171 | 0.998 |
| Wavenumber of Functional Group, cm−1 | Functional Group | Shifts in Relation to the FTIR Spectra, cm−1 | |||
|---|---|---|---|---|---|
| p(NiPAm-HPMet) | Piroxicam | p(NiPAm-HPMet) with Piroxicam | p(NiPAm-HPMet) | Piroxicam | |
| 3438 | 3449 | ν(OH) | +11 | ||
| 3319 | ν(NH) | − | |||
| 3393 | 3393 | ν(NH) | 0 | ||
| 3338 | 3346 | ν(OH) | +8 | ||
| 3066 | ν(C-H) pyridine | ||||
| 2973 | 2934 | 2973 | νas(CH3) | 0 | |
| 2933 | 2928 | νas(CH2) | −5 | −6 | |
| 2876 | 2857 | νs(CH3) | +19 | ||
| 1728 | 1742 | 1731 | νs (C=O) | +3 | −11 |
| 1650 | 1630 | 1636 | νs(C=O) amide I | −14 | +6 |
| 1560, 1474 | 1563, 1474 | ν(C=C) Ar | +3, 0 | ||
| 1576 | 1577 | ν(C-N) pyridine | +1 | ||
| 1544 | 1531 | 1531 | δ(NH) amide II | −13 | 0 |
| 1460 | 1436 | 1437 | δ(OH) | −23 | +1 |
| 1378 | δ(CN)- isopropyl | −10 | |||
| 1351 | 1351 | νas(SO2) | 0 | ||
| 1300 | 1300 | ν(C-N) amide III | 0 | ||
| 1182 | 1182 | νs(SO2) | 0 | ||
| 1173 | νas(C-O) | ||||
| 1131 | 1155 | 1156 | νs(C-O) | +25 | +1 |
| 925 | 939 | 939 | γ(OH) | +14 | 0 |
| 837 | 876, 831, 775 | 876, 831, 775 | δ(C-H) Ar | 0, 0, 0 | 0 |
| 732, 691 | γ(C-H) | ||||
| 674 | 692 | 669 | γ(OH) | −5 | - |
| p(NiPAm-HPMet) with mol% of Crosslinker EGDM | Lg Piroxicam (mg/gxerogel) | ηpiroxicam (%) |
|---|---|---|
| 1.0 | 331.35 | 66.34 |
| 1.5 | 273.39 | 54.79 |
| 2.0 | 234.45 | 47.08 |
| 3.0 | 191.83 | 38.45 |
| p(NiPAm-HPMet) with EGDM | mgpiroxicam/gxerogel | n | k (min−1/2) | R2 | D × 103 (cm2/min) | %, First 24 h | %, First 4 h |
|---|---|---|---|---|---|---|---|
| 1.0 mol% | 86.616 | 0.062 | 0.646 | 0.979 | 3.28 | 19.24 | 17.23 |
| 1.5 mol% | 75.246 | 0.099 | 0.543 | 0.988 | 3.32 | 18.81 | 17.31 |
| 2.0 mol% | 67.134 | 0.061 | 0.719 | 0.989 | 4.07 | 17.28 | 15.97 |
| 3.0 mol% | 65.133 | 0.054 | 0.948 | 0.986 | 4.14 | 22.97 | 21.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Biomedical Applications of Thermosensitive Hydrogels for Controlled/Modulated Piroxicam Delivery. Gels 2023, 9, 70. https://doi.org/10.3390/gels9010070
Ilić-Stojanović S, Nikolić L, Nikolić V, Ristić I, Cakić S, Petrović SD. Biomedical Applications of Thermosensitive Hydrogels for Controlled/Modulated Piroxicam Delivery. Gels. 2023; 9(1):70. https://doi.org/10.3390/gels9010070
Chicago/Turabian StyleIlić-Stojanović, Snežana, Ljubiša Nikolić, Vesna Nikolić, Ivan Ristić, Suzana Cakić, and Slobodan D. Petrović. 2023. "Biomedical Applications of Thermosensitive Hydrogels for Controlled/Modulated Piroxicam Delivery" Gels 9, no. 1: 70. https://doi.org/10.3390/gels9010070