Effects of Iron Minerals on the Compressive Strengths and Microstructural Properties of Metakaolin-Based Geopolymer Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of Starting Materials
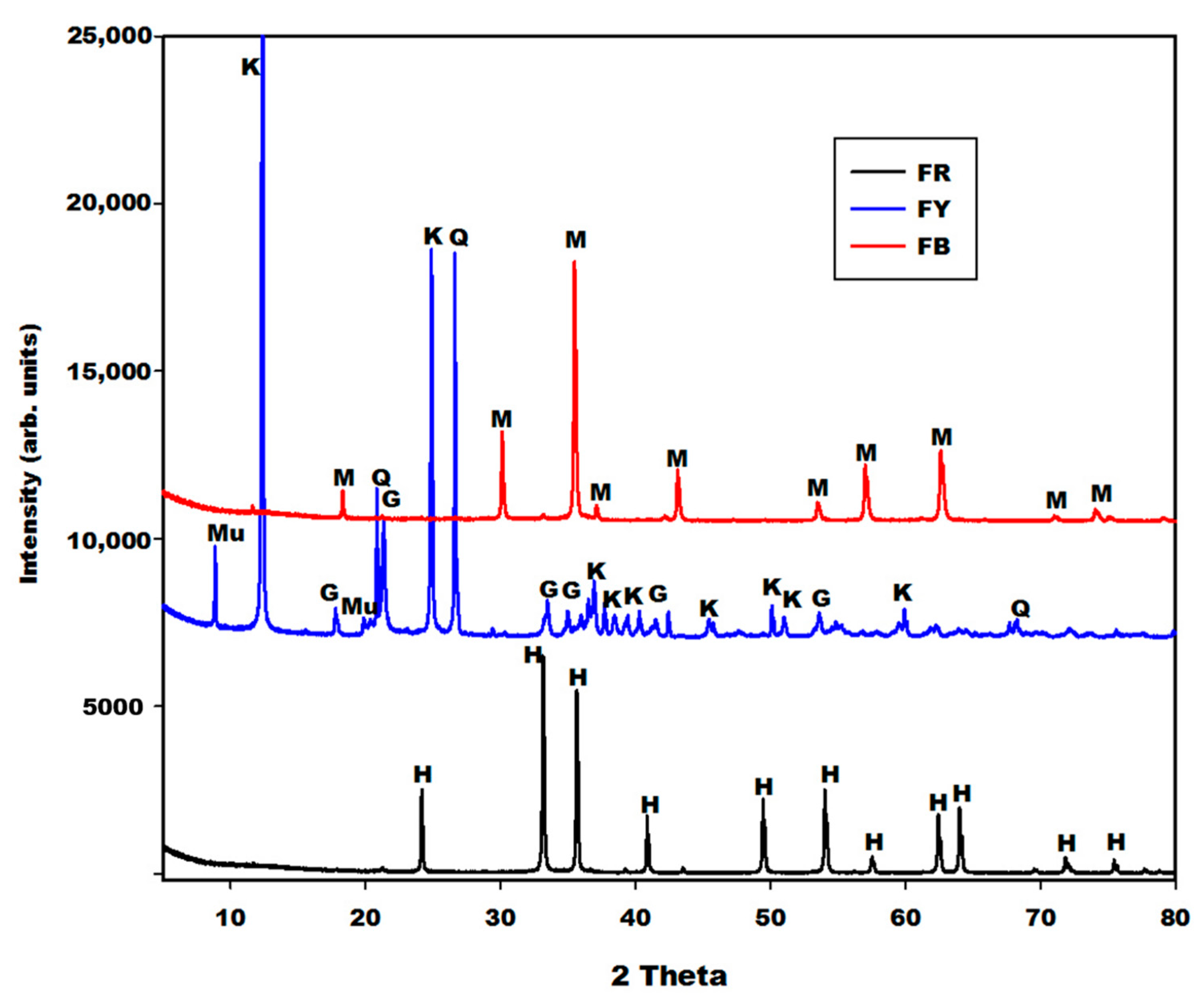
2.1.1. X-ray Patterns
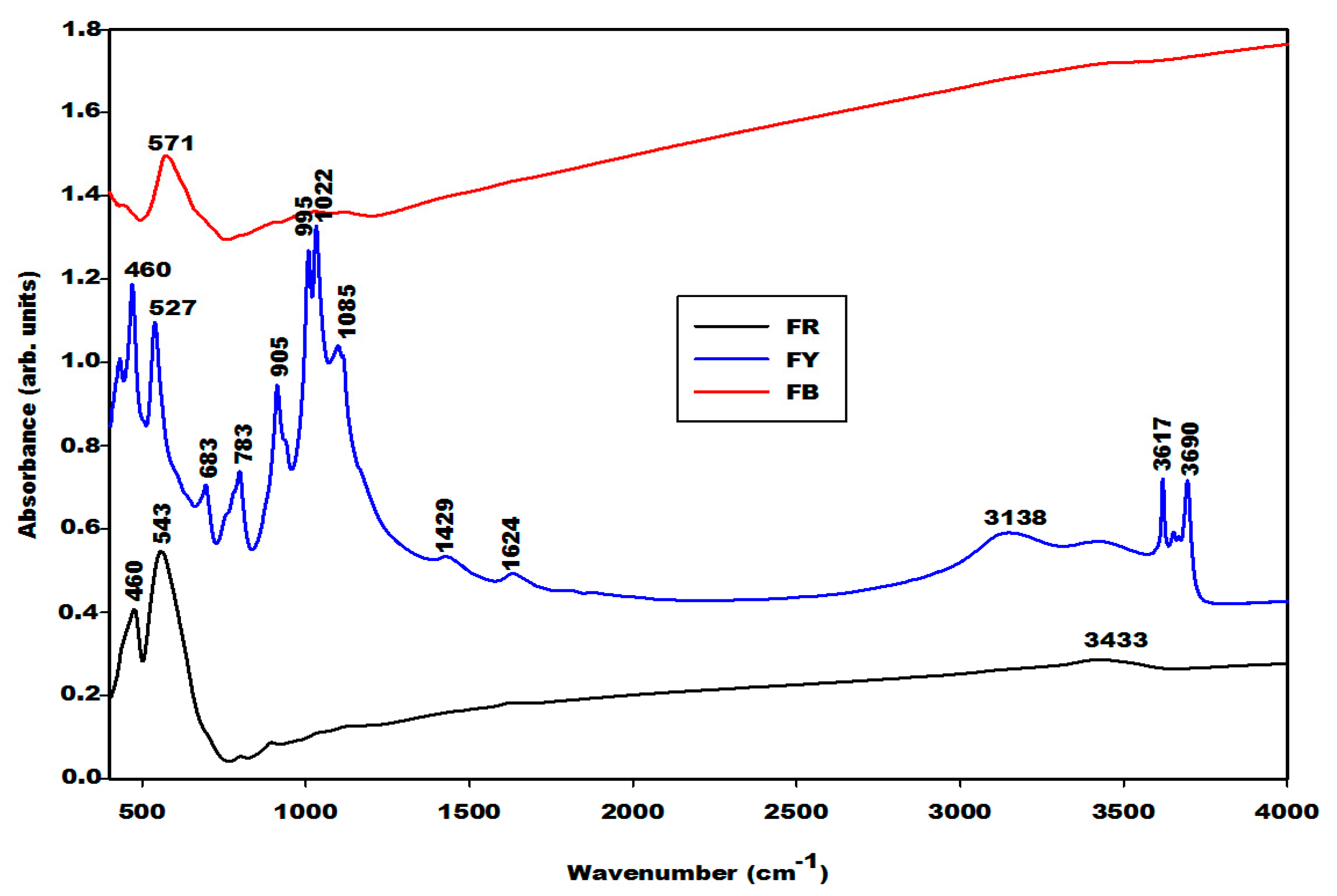
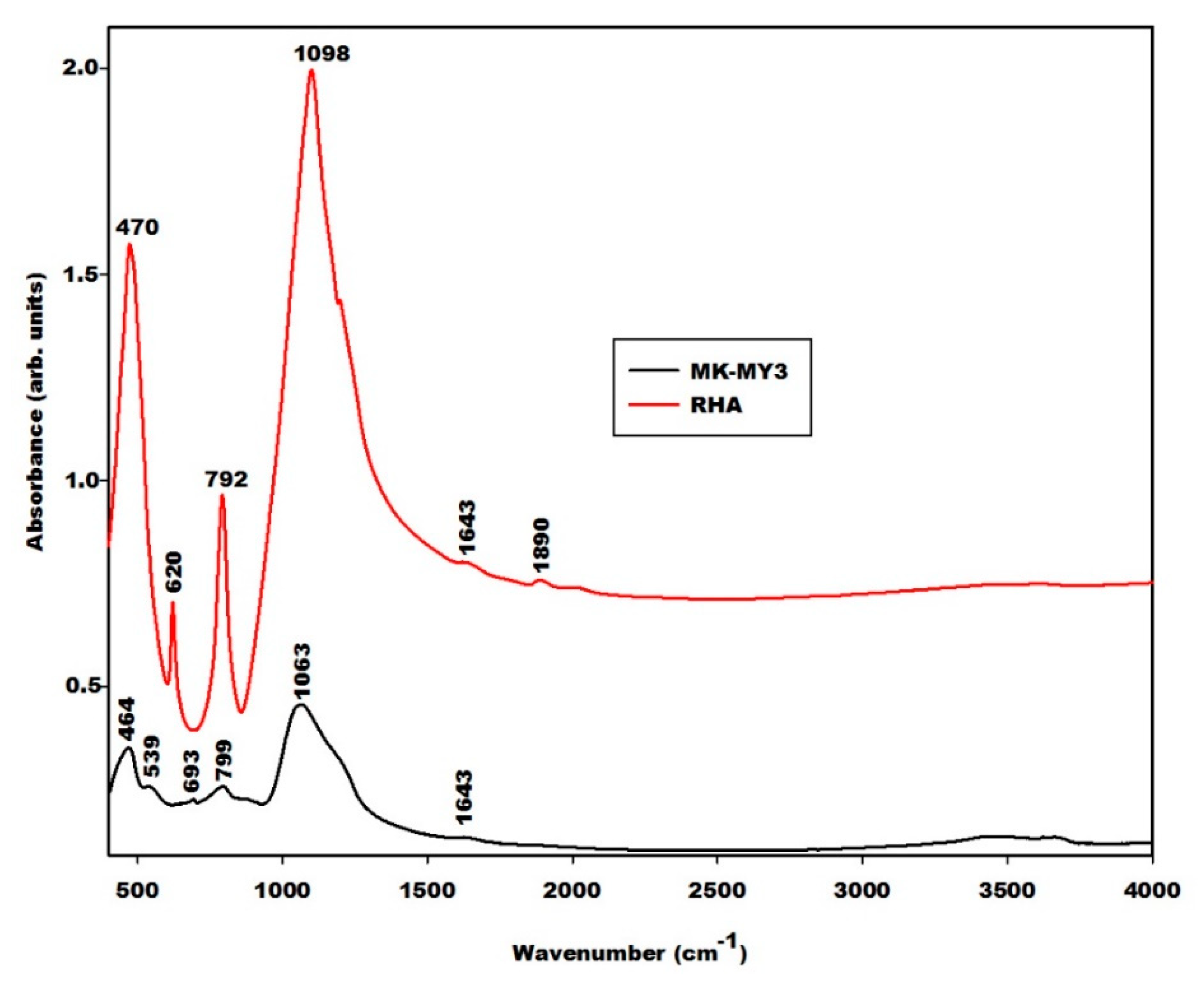
2.1.2. Infrared Spectra
2.2. Characterisation of Geopolymer Materials
2.2.1. X-ray Diffractograms, Crystalline and Amorphous Phases Content
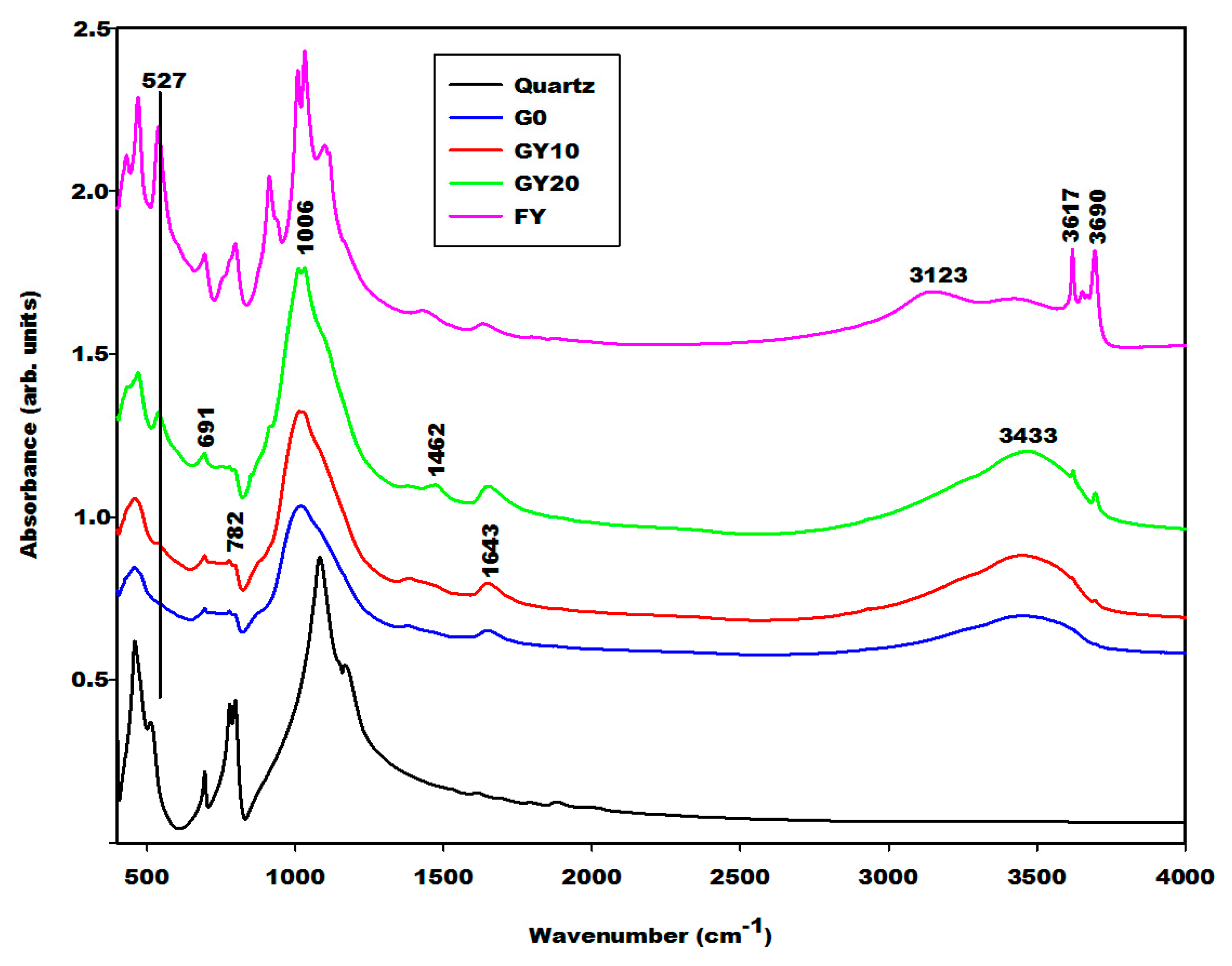
2.2.2. Infrared Spectra
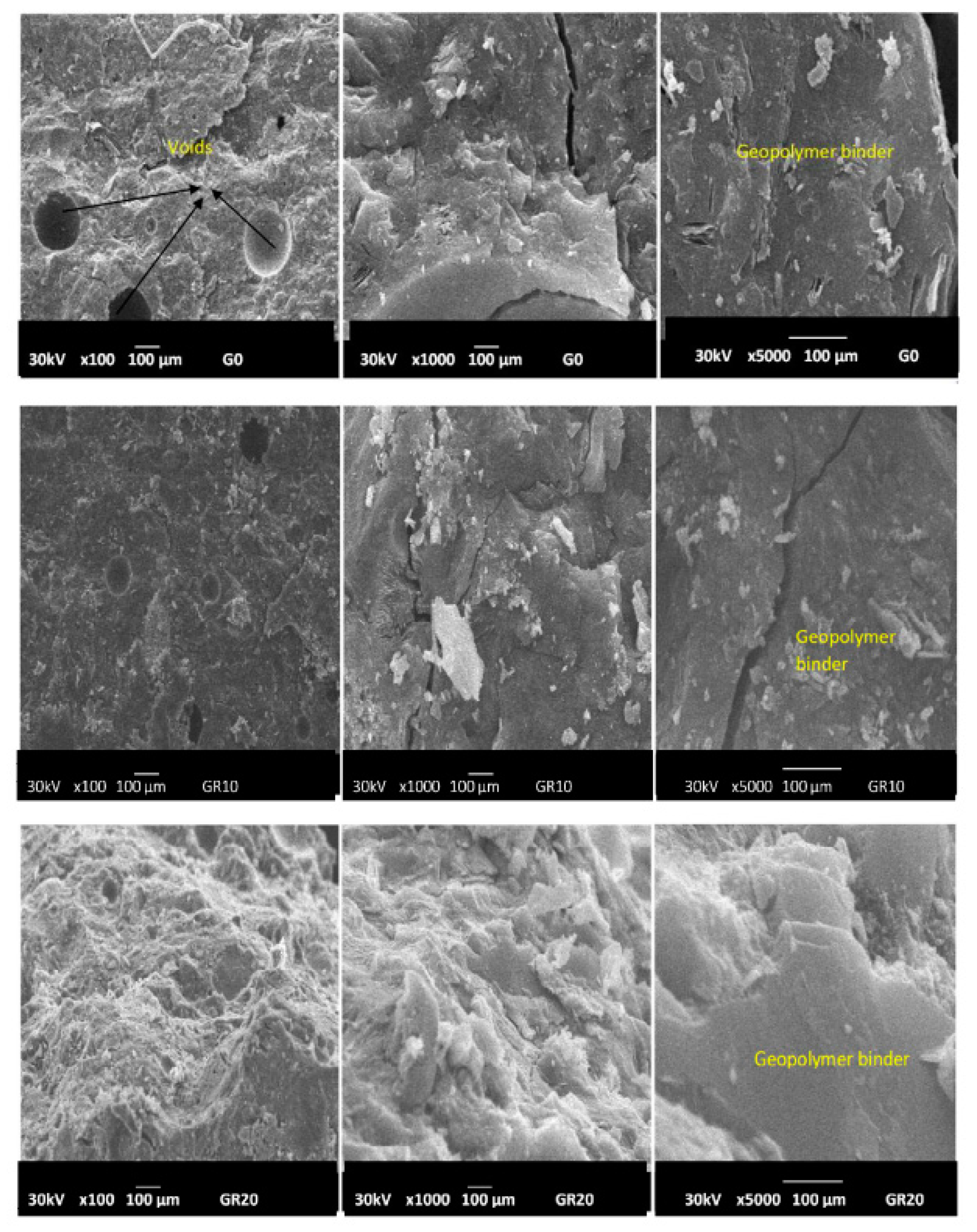
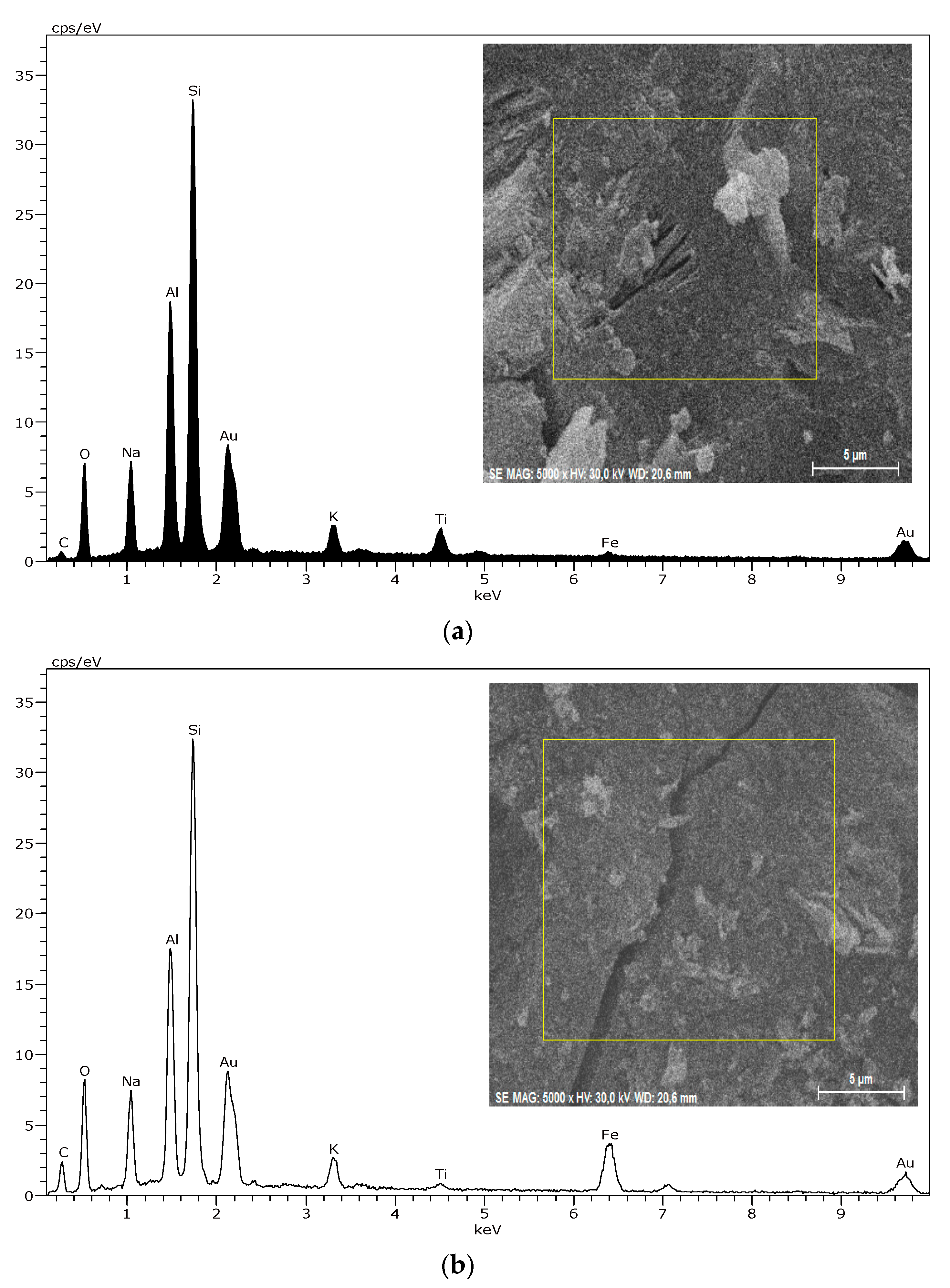
2.2.3. Micrography Image Investigations
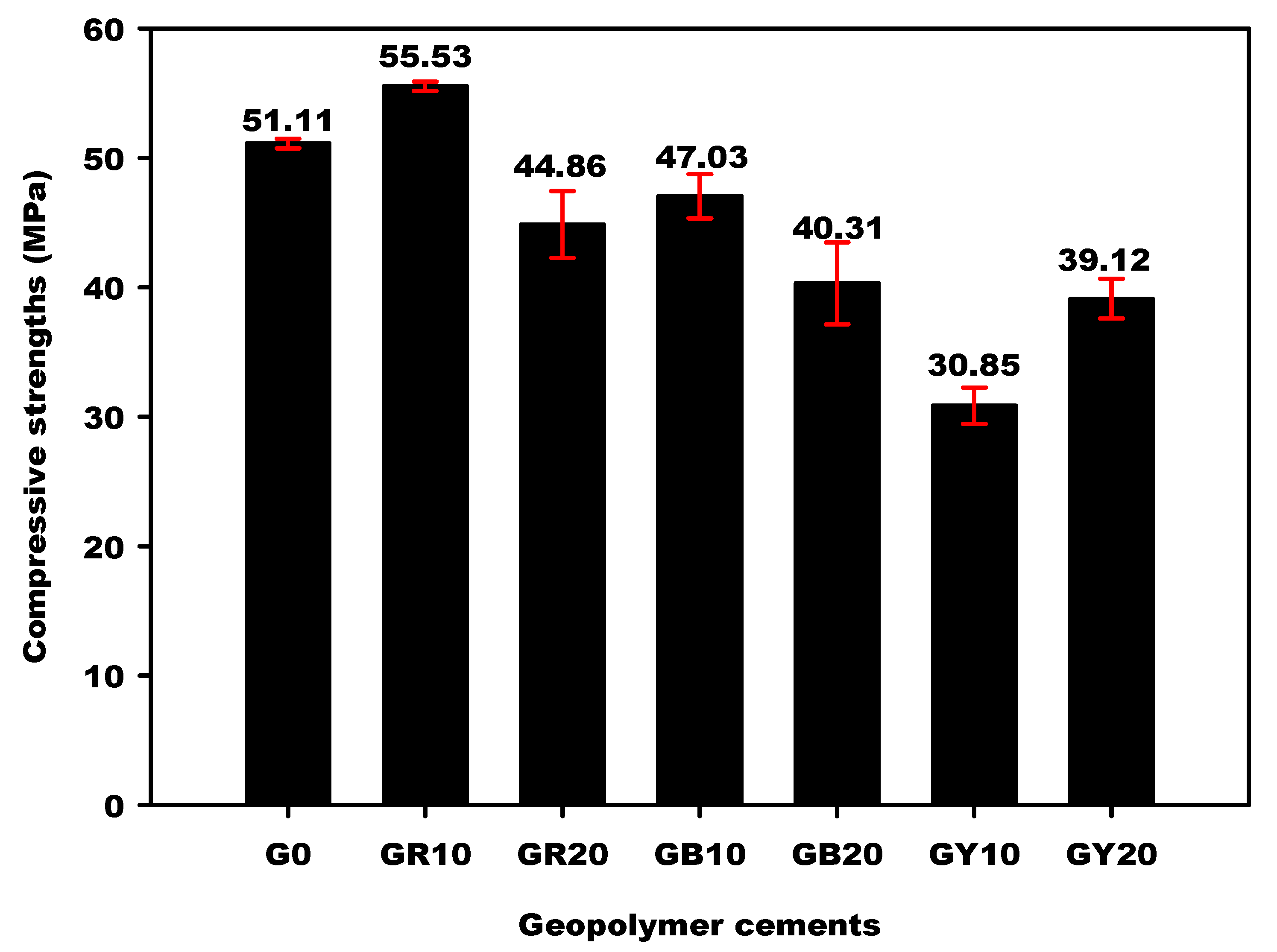
2.2.4. Compressive Strengths
3. Conclusions
4. Materials and Experimental Methods
4.1. Materials
| Samples | MY3 Njoya et al. (2006) | RHA Melele et al. (2019), Mabah et al. (2019) | |
|---|---|---|---|
| Oxides | |||
| MgO | / | 0.28 | |
| Al2O3 | 33.29 | 0.58 | |
| SiO2 | 46.61 | 93.20 | |
| K2O | 0.94 | 3.05 | |
| CaO | / | 0.57 | |
| TiO2 | 3.96 | 0.03 | |
| SO2 | 0.05 | / | |
| Fe2O3 | 1.46 | 2.20 | |
| P2O5 | 0.40 | / | |
| Others | / | 1.78 | |
| LOI | 13.97 | 1.2 | |
4.2. Experimental Methods
4.2.1. Preparation of Sodium Waterglass from Rice Husk Ash and Geopolymer Materials
4.2.2. Methods of Characterization of Raw Materials and Metakaolin-Based Geopolymer Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kani, E.N.; Rafiean, A.H.; Alishah, A.; Astani, S.H.; Ghaffar, S.H. The effects of Nano-Fe2O3 on the mechanical, physical and microstructure of cementitious composites. Constr. Build. Mater. 2021, 266, 121137. [Google Scholar] [CrossRef]
- Yazdi, N.A.; Arefi, M.R.; Mollaahmadi, E.; Nejand, B.A. To study the effect of adding Fe2O3 nanoparticles on the morphology properties and microstructure of cement mortar. Life Sci. J. 2011, 8, 550–554. [Google Scholar]
- Ghazanlou, S.I.; Jalaly, M.; Sadeghzadeh, S.; Korayem, A.H. A comparative study on the mechanical, physical and morphological properties of cement-micro/nano Fe3O4 composite. Sci. Rep. 2020, 10, 2859. [Google Scholar] [CrossRef] [PubMed]
- Essaidi, N.; Samet, B.; Baklouti, S.; Rossignol, S. The role of hematite in aluminosilicate gels based on metakaolin. Ceram.-Silik. 2014, 58, 1–11. [Google Scholar]
- Zailani, W.W.A.; Abdullah, M.M.A.; Arshad, M.F.; Burduhos-Nergis, D.D.; Tahir, M.F.M. Effect of iron oxide (Fe2O3) on the properties of fly ash based geopolymer. International Conference on Innovative Research—ICIR EUROINVENT 2020 IOP. IOP Conf. Ser. Mater. Sci. Eng. 2020, 877, 012017. [Google Scholar] [CrossRef]
- Kaya, M.; Koksal, F.; Gencel, O.; Munir, M.J.; Minhaj, S.; Kazmi, S. Influence of micro Fe2O3 and MgO on the physical and mechanical properties of the zeolite and kaolin based geopolymer mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Abdalla, J.A.; Thomas, B.S.; Hawileh, R.A.; Yang, J.; Jindal, B.B.; Ariyachandra, E. Influence of nano-TiO2, nano-Fe2O3, nanoclay and nano-CaCO3 on the properties of cement/geopolymer concrete. Clean. Mater. 2022, 4, 100061. [Google Scholar] [CrossRef]
- Samen, V.E.L.K.; Kaze, R.C.; Nemaleu, J.G.D.; Tchakoute, H.K.; Meukam, P.; Kamseu, E.; Leonelli, C. Engineering properties, phase evolution and microstructure of the iron-rich aluminosilicates-cement based composites: Cleaner production of energy efficient and sustainable materials. Cleaner Mater. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Fekoua, J.N.N.; Kaze, C.R.; Duna, L.L.; Ghazouni, A.; Ndassa, I.M.; Kamseu, E.; Rossignol, S.; Leonelli, C. Effects of curing cycles on developing strength and microstructure of goethite-rich aluminosilicate (corroded laterite) based geopolymer composites. Mater. Chem. Phys. 2021, 270, 124864. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers, Technical Papers # 27, Geopolymer Institute Library. 2020. Available online: https://www.geopolymer.org/news/27-ferro-sialate-geopolymers/ (accessed on 11 January 2020).
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Andrade, A.L.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. Synthesis and characterization of magnetic nanoparticles coated with silica through a sol-gel approach. Cerâmica 2009, 55, 420–424. [Google Scholar] [CrossRef]
- Deligöz, H.; Baykal, A.; Tanriverdi, E.; Durmus, Z.; Toprak, M.S. Synthesis, structural and electrical properties of triethylene glycol (TREG) stabilized Mn0.2Co0.8Fe2O4 NPs. Mater. Res. Bull. 2012, 47, 537–543. [Google Scholar] [CrossRef]
- Srivastava, S. Synthesis and characterization of iron oxide nanoparticle from FeCl3 by using polyvinyl alcohol. Inter. J. Phys. Soc. Sci. 2012, 2, 161–184. [Google Scholar]
- Cui, H.; Ren, W.; Lin, P.; Liu, Y. Structure control synthesis of iron oxide polymorph nanoparticles through an epoxide precipitation route. J. Exp. Nanosci. 2013, 8, 869–875. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared spectroscopic characterization of kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef]
- Prasetyoko, D.; Ramli, Z.; Endud, S.; Hamdan, H.; Sulikowski, B. Conversion of rice husk ash to zeolite beta. Waste Manag. 2006, 26, 1173–1179. [Google Scholar] [CrossRef]
- Tang, C.; Zhu, J.; Lid, Z.; Zhu, R.; Zhou, Q.; Wei, J.; He, H.; Tao, Q. Surface chemistry and reactivity of SiO2 polymorphs: A comparative study on α-quartz and α-cristobalite. Appl. Surf. Sci. 2015, 355, 1161–1167. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 8, 967–981. [Google Scholar] [CrossRef]
- Moreno, E.M.; Zayat, M.; Morales, M.P.; Serna, C.J.; Roig, A.; Levy, D. Preparation of narrow size distribution superparamagnetic γ-Fe2O3 nanoparticles in a sol-gel transparent SiO2 matrix. Langmuir 2002, 18, 4972–4978. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Djobo, J.N.Y.; Kenne, B.B.D.; Njopwouo, D. Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl. Clay Sci. 2015, 107, 188–194. [Google Scholar] [CrossRef]
- Kaze, R.; Beleuk à Moungam, L.; Djouka, M.F.; Nana, A.; Kamseu, E.; Melo, U.C.; Leonelli, C. The corrosion of kaolinite by iron minerals and the effects on geopolymerization. Appl. Clay Sci. 2017, 138, 48–62. [Google Scholar] [CrossRef]
- Kaze, R.; Beleuk à Moungam, L.; Cannio, M.; Rosa, R.; Kamseu, E.; Chinje, U.; Leonelli, C. Microstructure and engineering properties of Fe2O3(FeO)-Al2O3-SiO2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Nkwaju, R.; Djobo, J.; Nouping, J.; Huisken, P.; Deutou, J.; Courard, L. Iron-rich laterite-bagasse fibers based geopolymer composite: Mechanical, durability and insulating properties. Appl. Clay Sci. 2019, 183, 105333. [Google Scholar] [CrossRef]
- Mimboe, A.G.; Abo, M.T.; Djobo, J.N.Y.; Tome, S.; Kaze, R.C.; Deutou, J.G.N. Lateritic soil based-compressed earth bricks stabilized with phosphate binder. J. Build. Eng. 2020, 31, 101465. [Google Scholar] [CrossRef]
- Messaoud, I.B.; Hamdi, N.; Srasra, E. Physicochemical characterization of geopolymer binders and foams made from Tunisian clay. Adv. Mater. Sci. Eng. 2018, 2018, 9392743. [Google Scholar] [CrossRef]
- Hong, S.B.; Woo, S.I.; Uh, Y.S. Crystallization of ferrisilicate molecular sieve in the presence of fluoride ions. Korean J. Chem. Eng. 1989, 6, 357–359. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Guo, Z.; Qin, J.; Liang, K. Effect of Fe2+/Fe3+ on high-strength ceramsite prepared by sintering geopolymers using iron ore tailings. Ceram. Inter. 2022, 48, 5681–5688. [Google Scholar] [CrossRef]
- Choi, S.-C.; Lee, W.-K. Effect of Fe2O3 on the physical property of geopolymer paste. Adv. Mater. Res. 2012, 586, 126–129. [Google Scholar] [CrossRef]
- Hawa, A.; Tonnayopas, D.; Prachasaree, W. Performance evaluation and microstructure characterization of metakaolin-based geopolymer containing oil Palm ash. Sci. World J. 2013, 2013, 857586. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, M.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vasquez, E.; et al. Environmental, physical and structural characterization of geopolymer matrixes synthesized from coal(co)-combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Al Kakni, A.M.M.; Kamaradin, H.; Bnhussian, M.; Rafiza, A.R.; Zarina, Y. Effect of Na2SiO3/NaOH molarities on compressive strength of fly ash-based geopolymer. ACF Mater. J. 2012, 109, 503–508. [Google Scholar]
- Dan, S.P.; Cashion, J.D.; Blackford, M.G.; Zhang, Z.; Vance, E.R. Fe speciation in geopolymers with Si/Al molar ratio of ~2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar]
- Lemougna, P.N.; Mackenzie, K.J.D.; Rahier, H.; Melo, U.F.C. The role of iron in the formation of inorganic polymers (geopolymers) from volcanic ash: A Fe Mössbauer spectroscopy study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Liao, K.Y.; Chang, P.K.; Peng, Y.N.; Yang, C.C. A study on characteristics of interfacial transition zone in concrete. Cem. Concr. Res. 2004, 34, 977–989. [Google Scholar] [CrossRef]
- Heikal, M.; Ibrahim, N.S. Hydration, microstructure and phase composition of composite cements containing nano-clay. Constr. Build. Mater. 2016, 1, 19–27. [Google Scholar] [CrossRef]
- Sadeghi-Nik, A.; Berenjian, J.; Bahari, A.; Safaei, A.S.; Dehestani, M. Modification of microstructure and mechanical properties of cement by nanoparticles through a sustainable development approach. Constr. Build. Mater. 2017, 30, 880–891. [Google Scholar] [CrossRef]
- Njoya, A.; Nkoumbou, C.; Grosbois, C.; Njopwouo, D.; Njoya, D.; Courtin-Nomade, A.; Yvon, J.; Martin, F. Genesis of Mayouom kaolin deposit (Western Cameroon). Appl. Clay Sci. 2006, 32, 125–140. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Ranjbar, N. Synthesis of sodium waterglass from white rice husk ash as an activator to produce metakaolin-based geopolymer cements. J. Build. Eng. 2016, 6, 252–261. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Mabah, D.E.T.; Rüscher, C.H.; Kamseu, E.; Andreola, F.; Bignozzi, M.C.; Leonelli, C. Preparation of low-cost nano and microcomposites from chicken eggshell, nano-silica and rice husk ash and their utilisations as additives for producing geopolymer cements. J. Asian Ceram. Soc. 2020, 8, 149–161. [Google Scholar] [CrossRef]
- Melele, S.J.K.; Banenzoué, C.; Fotio, D.; Tchakouté, H.K.; Rüscher, C.H.; Nanseu, C.P.N. Improvement of the reactivity of soda–lime–silica glass solution as a hardener for producing geopolymer materials. SN Appl. Sci. 2019, 1, 1208. [Google Scholar] [CrossRef]
- Mabah, D.E.T.; Tchakouté, H.K.; Fotio, D.; Rüscher, C.H.; Kamseu, E.; Bignozzi, M.C.; Leonelli, C. Influence of the molar ratios CaO/SiO2 contained in the sustainable microcomposites on the mechanical and microstructural properties of (Ca, Na)-poly(sialate-siloxo) networks. Mater. Chem. Phys. 2019, 238, 121928. [Google Scholar] [CrossRef]

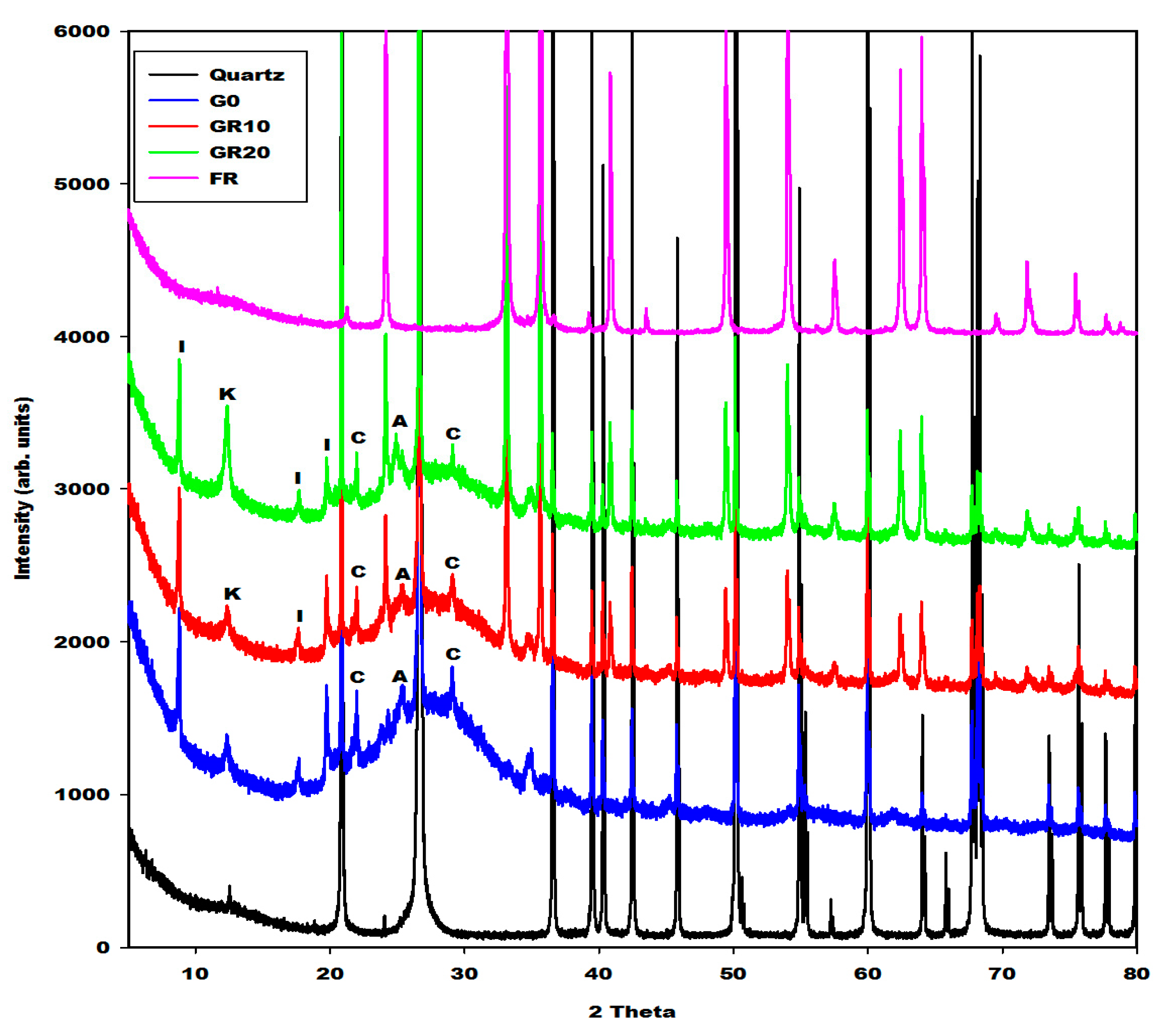
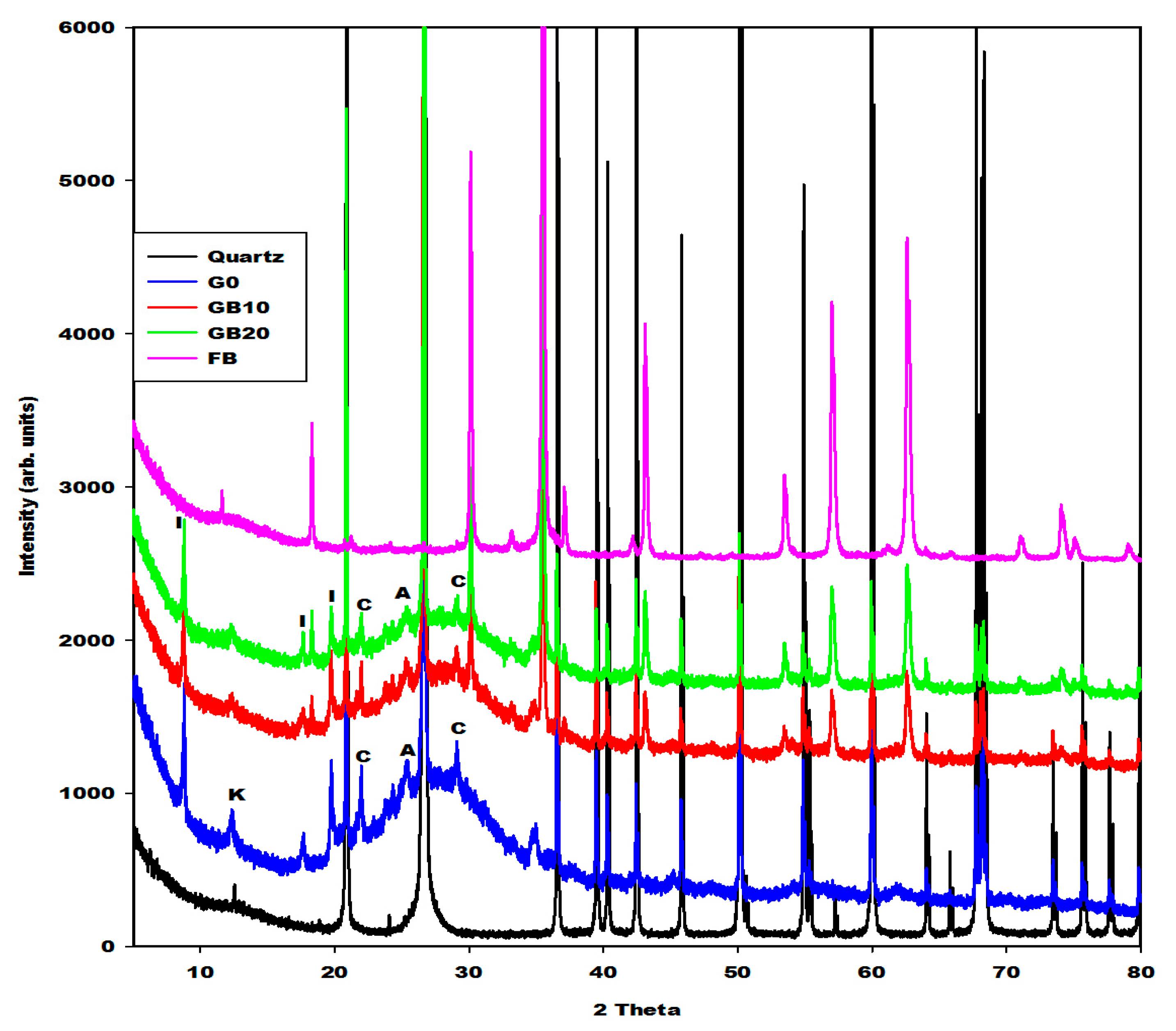
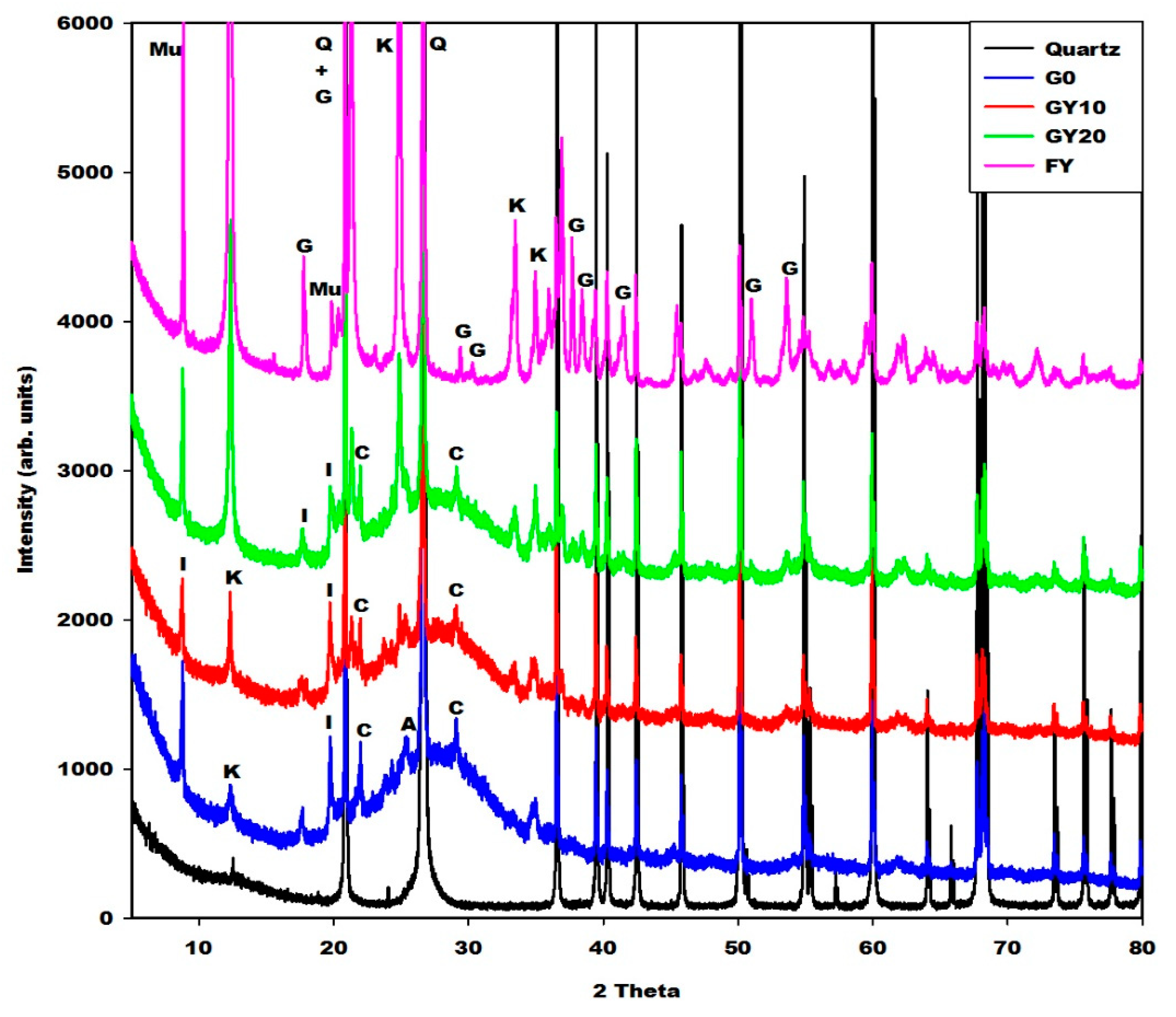
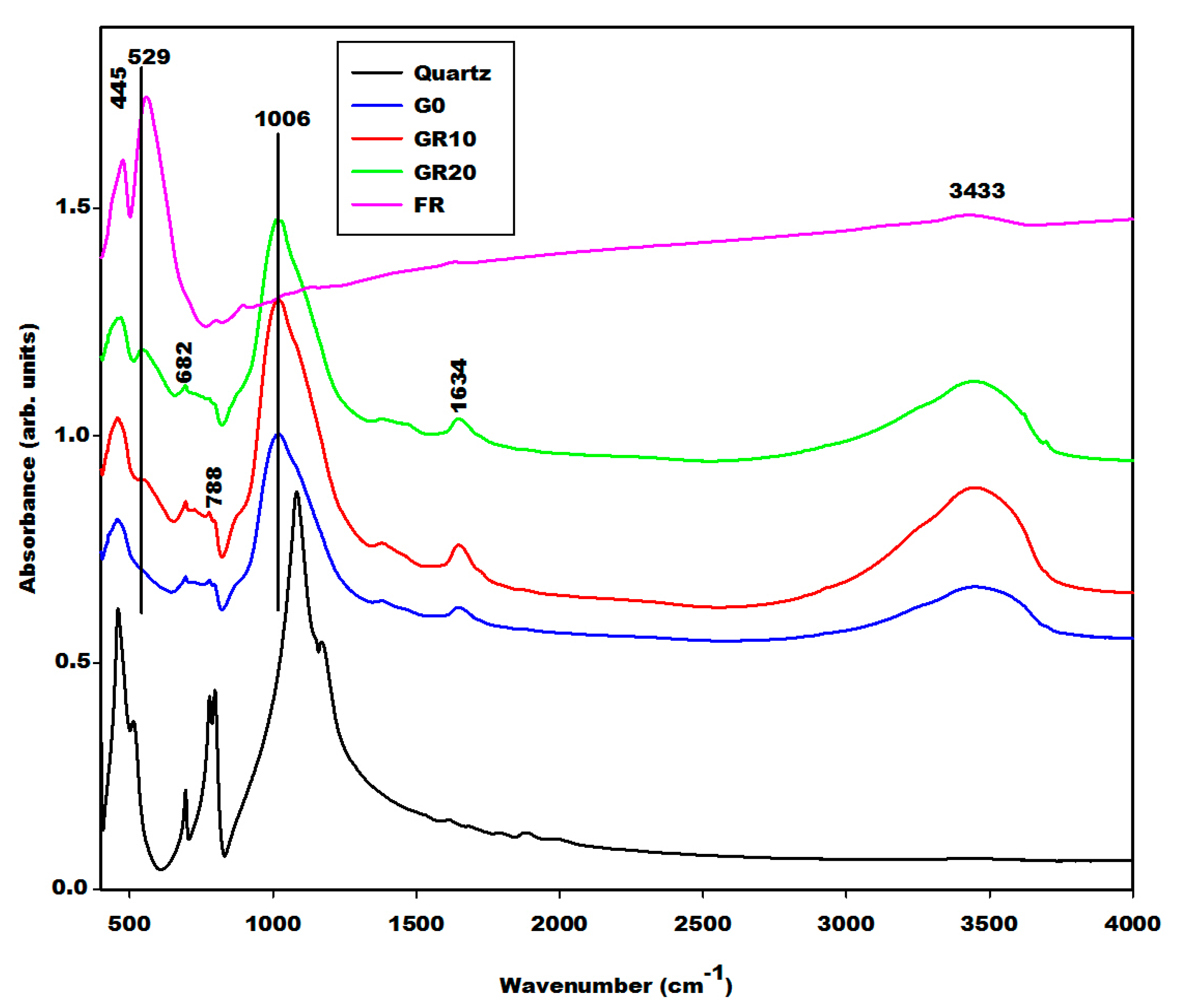
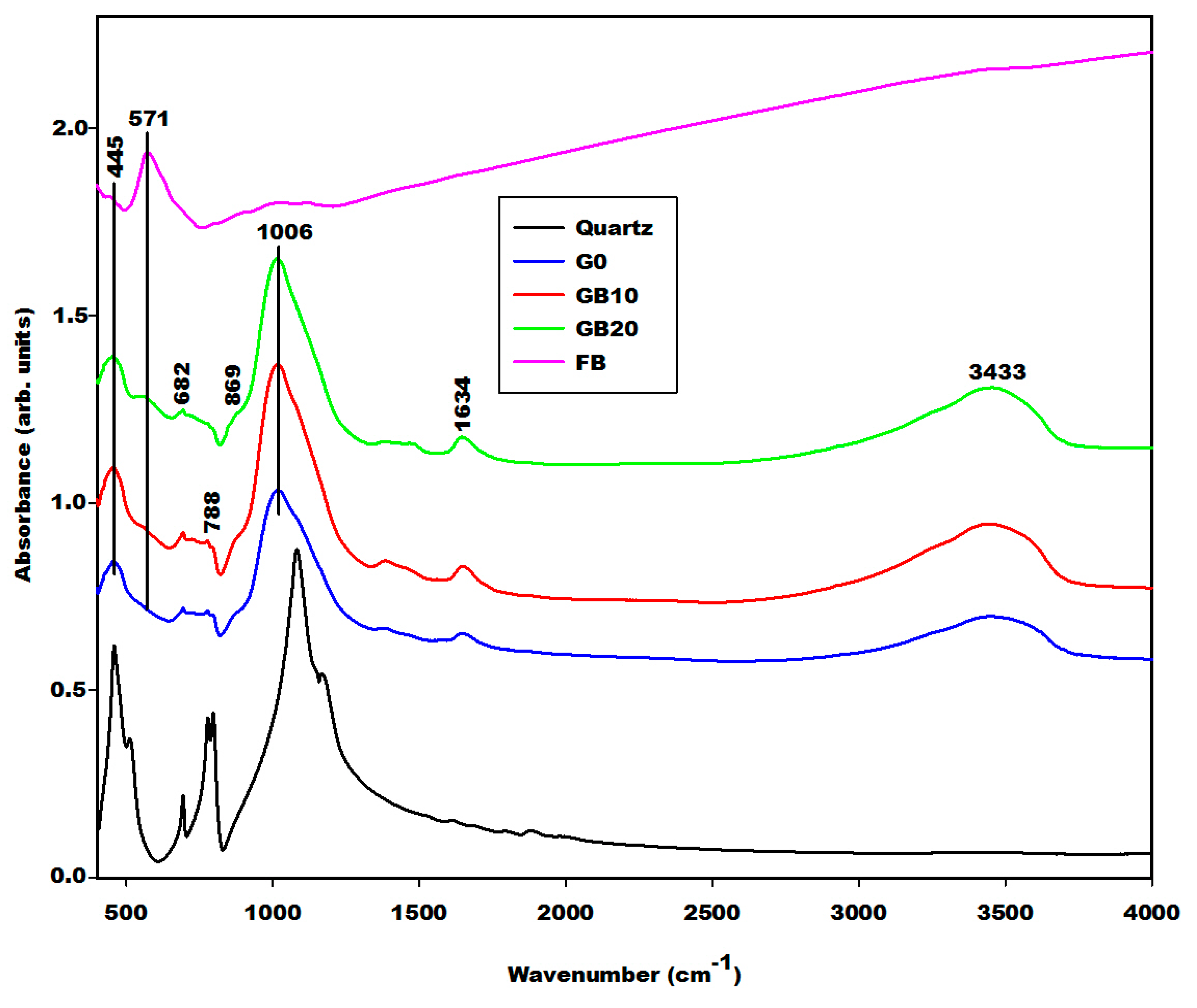



| Specimens | Amorphous Phase Contents (wt.%) | Crystalline Phase Contents (wt.%) |
|---|---|---|
| G0 | 49.4 | 50.6 |
| GR10 | 38.6 | 61.4 |
| GR20 | 35.4 | 64.6 |
| GB10 | 43.5 | 56.5 |
| GB20 | 39.9 | 60.1 |
| GY10 | 45.1 | 54.9 |
| GY20 | 42.0 | 58.0 |
| Specimens | O2 | Na | Al | Si | Ti | K | Fe |
|---|---|---|---|---|---|---|---|
| G0 | 60.57 | 8.00 | 10.89 | 19.60 | 0.34 | 0.52 | 0.08 |
| GR10 | 60.49 | 7.32 | 11.41 | 17.78 | 1.34 | 0.37 | 1.27 |
| GR20 | 57.44 | 11.07 | 8.81 | 16.18 | 0.08 | 0.51 | 5.93 |
| GB10 | 59.08 | 10.06 | 9.10 | 18.92 | 0.02 | 0.60 | 2.21 |
| GB20 | 60.55 | 6.45 | 5.74 | 21.61 | 0.0 | 0.29 | 5.38 |
| GY10 | 60.83 | 6.75 | 11.79 | 19.18 | 0.25 | 0.56 | 0.62 |
| GY20 | 60.67 | 6.13 | 8.95 | 20.23 | 0.16 | 0.90 | 2.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngnintedem, D.L.V.; Lampe, M.; Tchakouté, H.K.; Rüscher, C.H. Effects of Iron Minerals on the Compressive Strengths and Microstructural Properties of Metakaolin-Based Geopolymer Materials. Gels 2022, 8, 525. https://doi.org/10.3390/gels8080525
Ngnintedem DLV, Lampe M, Tchakouté HK, Rüscher CH. Effects of Iron Minerals on the Compressive Strengths and Microstructural Properties of Metakaolin-Based Geopolymer Materials. Gels. 2022; 8(8):525. https://doi.org/10.3390/gels8080525
Chicago/Turabian StyleNgnintedem, Dimace Lionel Vofo, Marco Lampe, Hervé Kouamo Tchakouté, and Claus Henning Rüscher. 2022. "Effects of Iron Minerals on the Compressive Strengths and Microstructural Properties of Metakaolin-Based Geopolymer Materials" Gels 8, no. 8: 525. https://doi.org/10.3390/gels8080525





