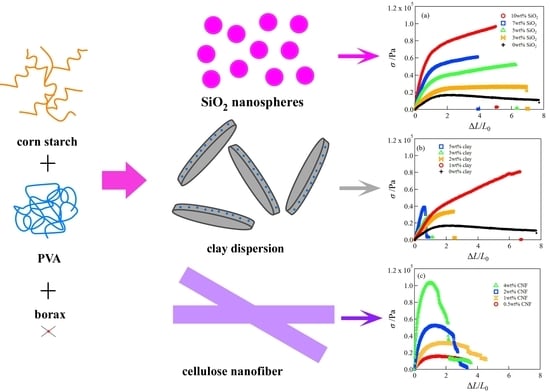
Mechanical Performance of Corn Starch/Poly(Vinyl Alcohol) Composite Hydrogels Reinforced by Inorganic Nanoparticles and Cellulose Nanofibers
Abstract
:1. Introduction
2. Results and Discussion
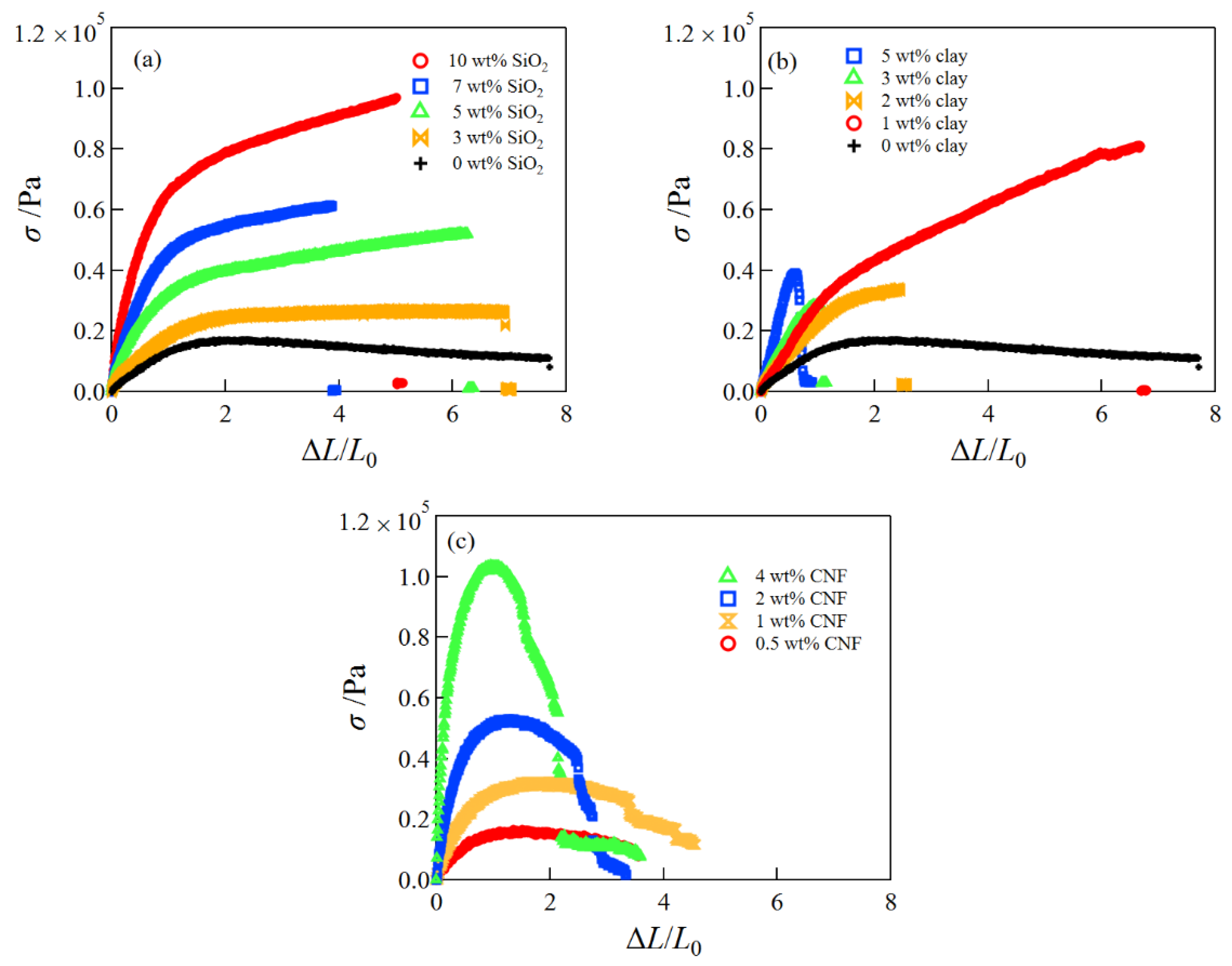
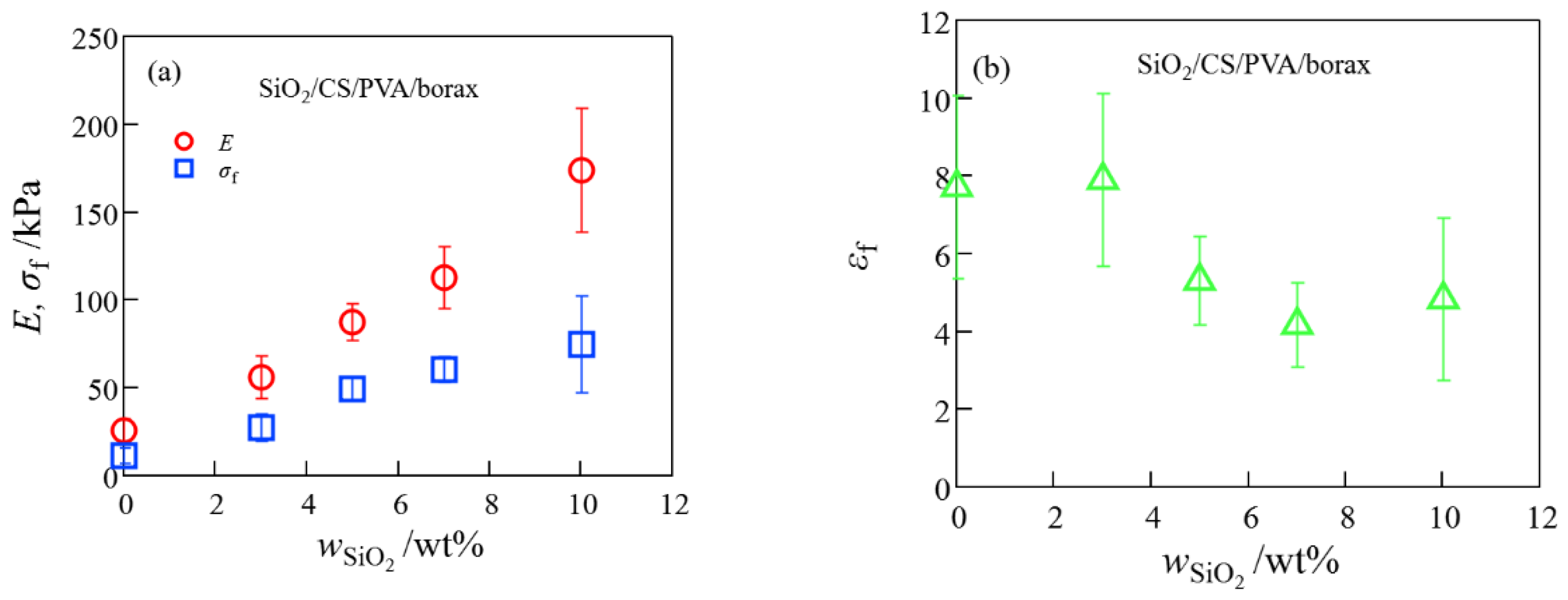
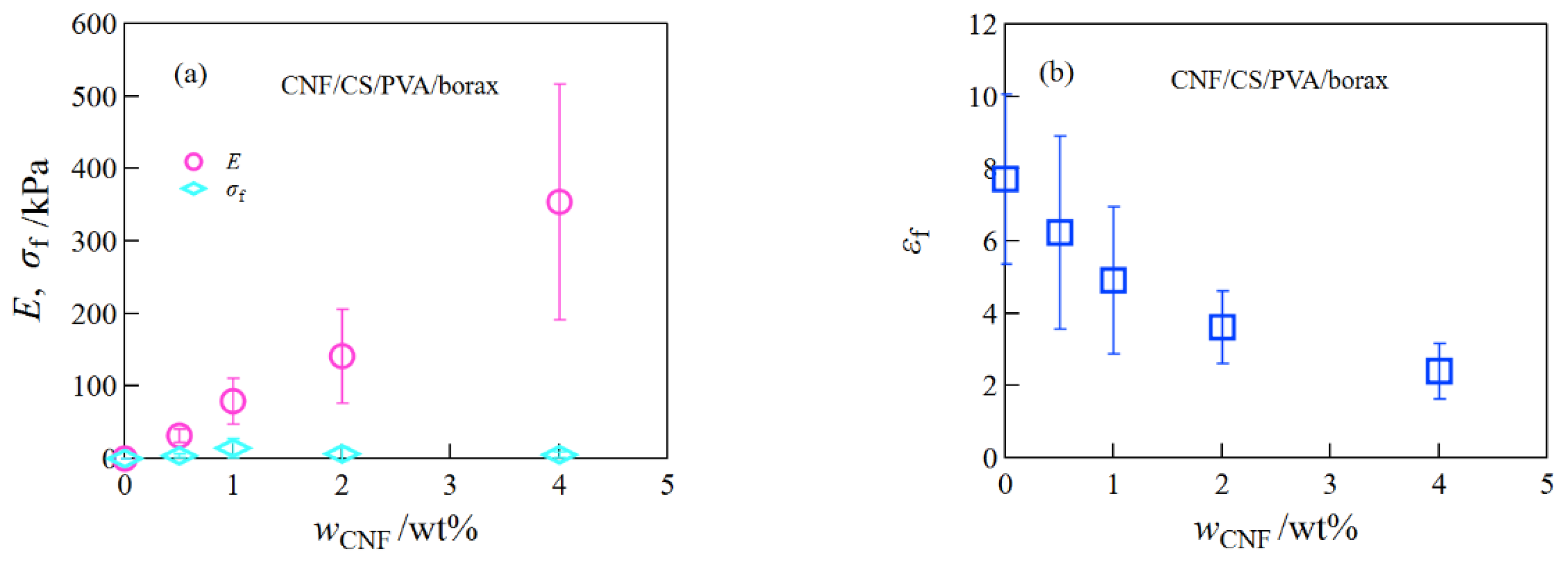
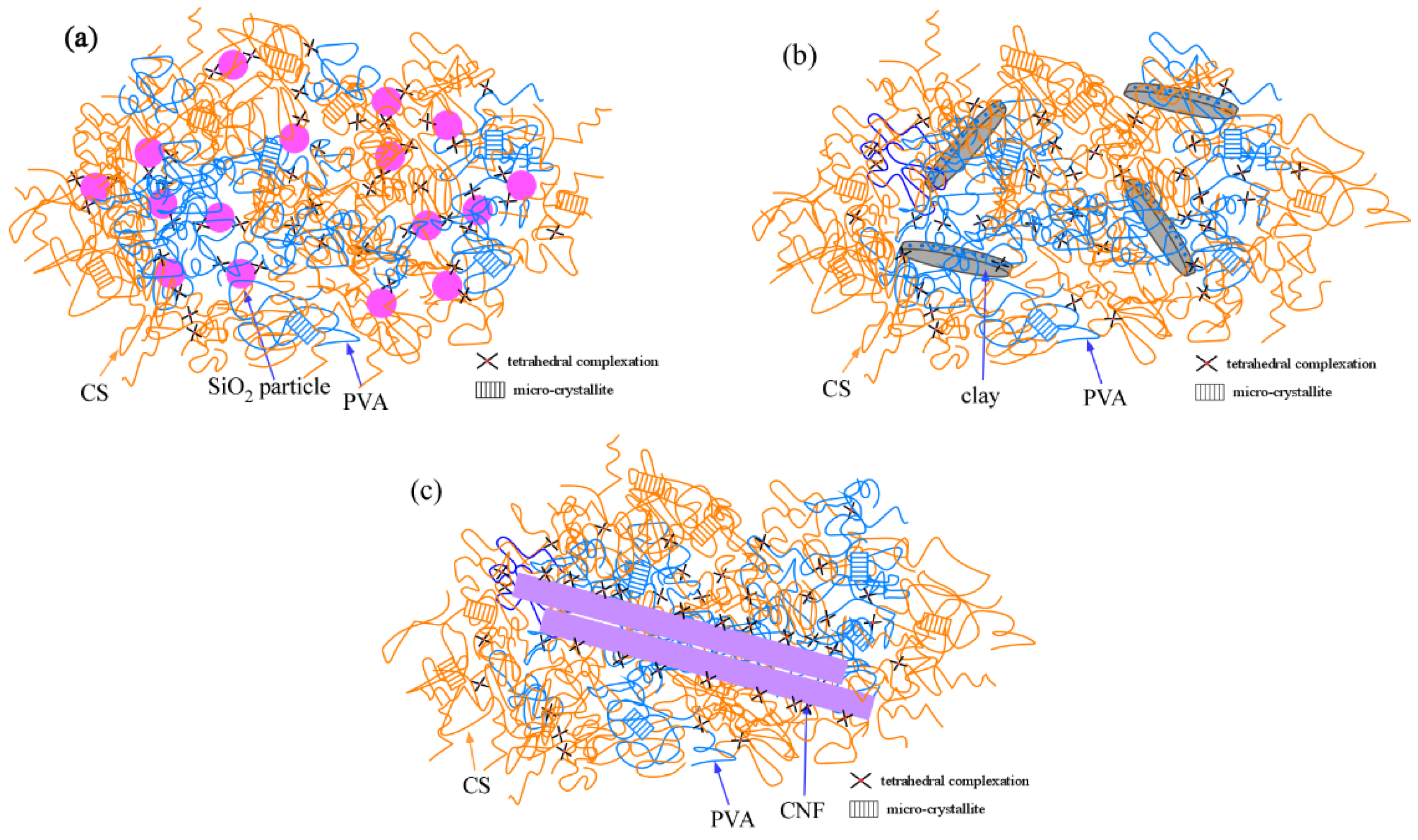
2.1. Mechanical Properties of SiO2/CS/PVA/Borax, Clay/CS/PVA/Borax, and CNF/CS/PVA/Borax Composite Hydrogels
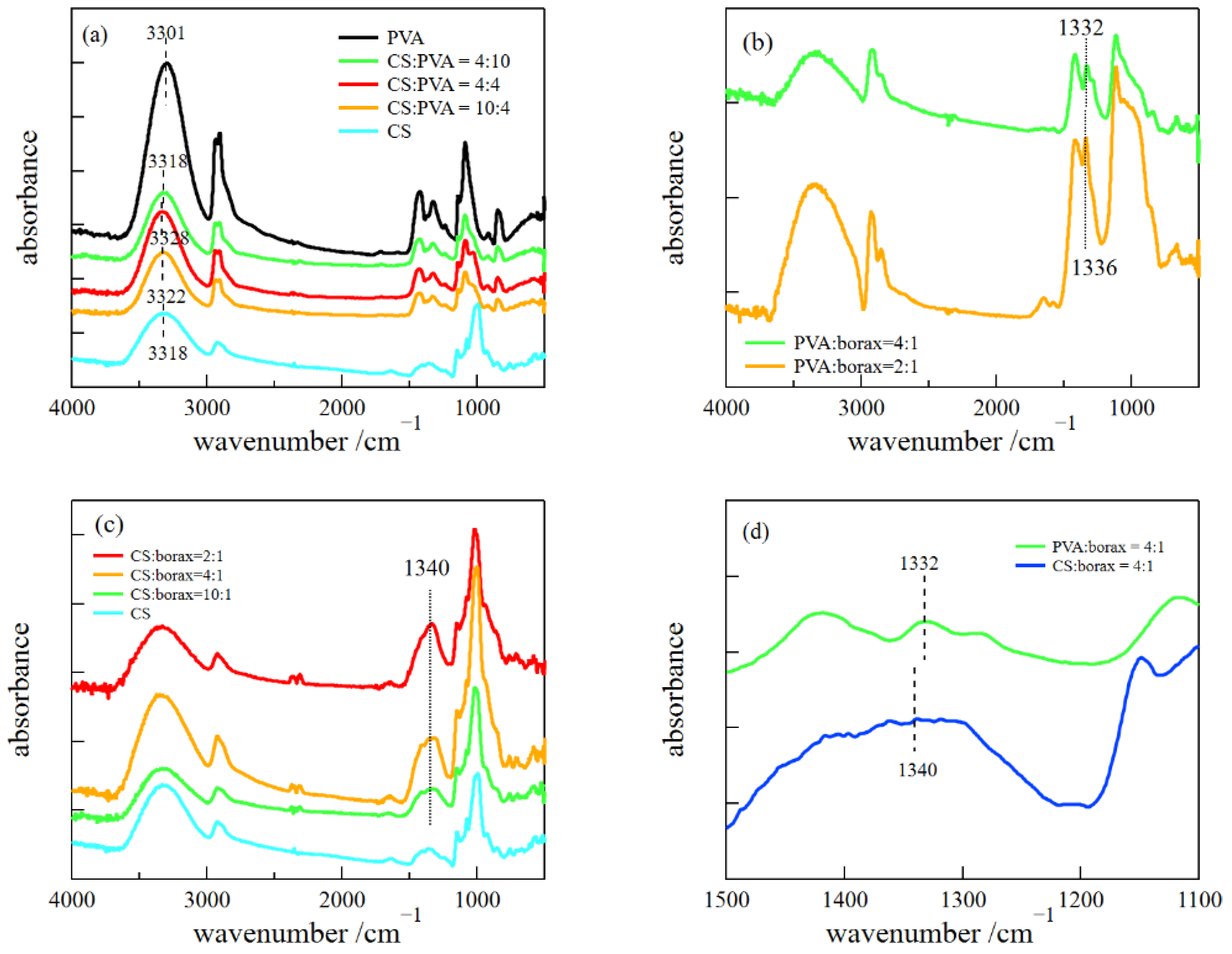
2.2. FT-IR Measurements
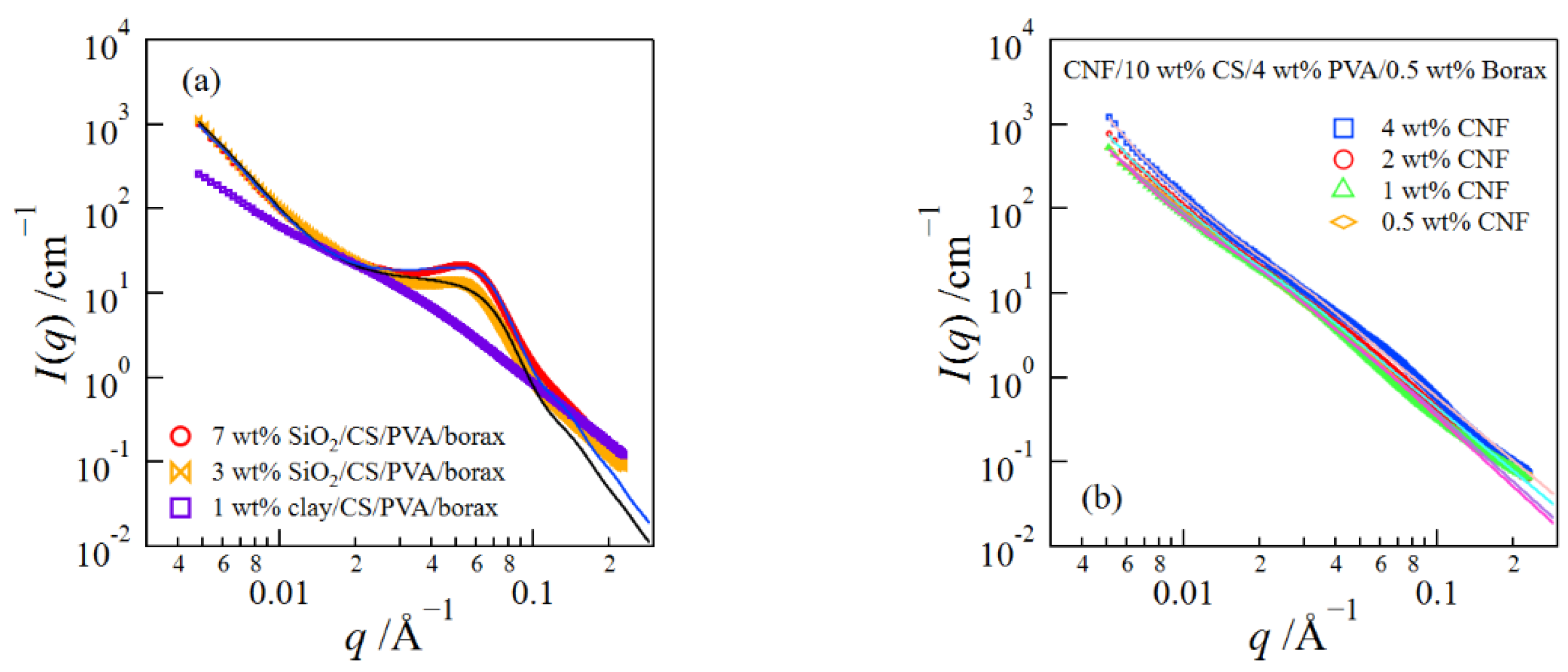
2.3. Synchrotron SAXS/WAXS Measurements
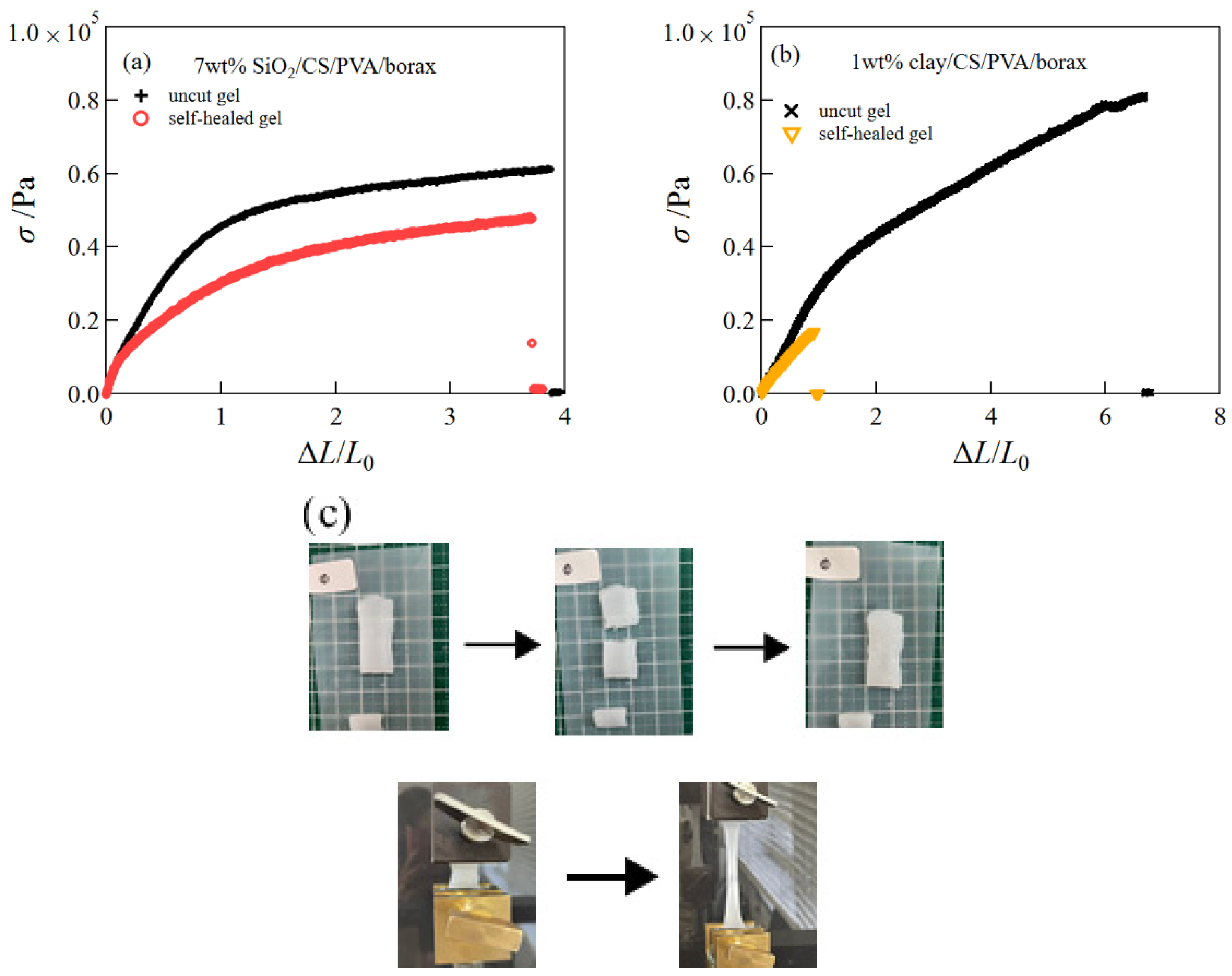
2.4. Self-Healing
2.5. Comparison of Mechanical Properties of SiO2/CS/PVA/Borax, Clay/CS/PVA/Borax, and CNF/CS/PVA/Borax Composite Hydrogels
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of Composite Hydrogels
4.3. Tensile Measurements
4.4. Fourier Transform Infrared Spectroscopy (FT-IR)
4.5. Synchrotron Simultaneous Small-Angle X-ray Scattering (SAXS)/Wide-Angle X-ray Scattering (WAXS) Measurement
4.6. Self-Healing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonenc, I.; Us, F. Effect of Glutaraldehyde Crosslinking on Degree of Substitution, Thermal, Structural, and Physicochemical Properties of Corn Starch. Starch-Stärke 2019, 71, 1800046. [Google Scholar] [CrossRef]
- Xiao, C. Current advances of chemical and physical starch-based hydrogels. Starch-Stärke 2013, 65, 82–88. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.; Qiu, C.; Xu, X.; Jin, Z. A Dual Cross-Linked Strategy to Construct Moldable Hydrogels with High Stretchability, Good Self-Recovery, and Self-Healing Capability. J. Agric. Food. Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef]
- Takeno, H.; Kimura, Y.; Nakamura, W. Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels 2017, 3, 10. [Google Scholar] [CrossRef]
- Takeno, H.; Nakamura, W. Structural and mechanical properties of composite hydrogels composed of clay and a polyelectrolyte prepared by mixing. Colloid. Polym. Sci. 2013, 291, 1393–1399. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, K.; Zhou, D.; Yang, H.; Liu, X.; Yin, X.; Xu, W.; Zhou, Y.; Xiao, P. High-Performance Photopolymerized Poly(vinyl alcohol)/Silica Nanocomposite Hydrogels with Enhanced Cell Adhesion. Acs Appl. Mater. Inter. 2018, 10, 27692–27700. [Google Scholar] [CrossRef]
- Takeno, H.; Suto, N. Robust and Highly Stretchable Chitosan Nanofiber/Alumina-Coated Silica/Carboxylated Poly (Vinyl Alcohol)/Borax Composite Hydrogels Constructed by Multiple Crosslinking. Gels 2022, 8, 6. [Google Scholar] [CrossRef]
- Boyaci, T.; Orakdogen, N. Poly(N,N-dimethylaminoethyl methacrylate-co-2-acrylamido-2-methyl-propanosulfonic acid)/Laponite nanocomposite hydrogels and cryogels with improved mechanical strength and rapid dynamic properties. Appl. Clay Sci. 2016, 121–122, 162–173. [Google Scholar] [CrossRef]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(N,N-dimethylacrylamide) and clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide-clay nanocomposite hydrogels: Rheological and light scattering characterization. Macromolecules 2007, 40, 3378–3387. [Google Scholar] [CrossRef]
- Takeno, H.; Sato, C. Effects of molecular mass of polymer and composition on the compressive properties of hydrogels composed of Laponite and sodium polyacrylate. Appl. Clay Sci. 2016, 123, 141–147. [Google Scholar] [CrossRef]
- Takeno, H.; Aoki, Y.; Kimura, K. Effects of silica and clay nanoparticles on the mechanical properties of poly(vinyl alcohol) nanocomposite hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127592. [Google Scholar] [CrossRef]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanical and structural properties of cellulose nanofiber/poly(vinyl alcohol) hydrogels cross-linked by a freezing/thawing method and borax. Cellulose 2020, 27, 4373–4387. [Google Scholar] [CrossRef]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanically robust ionic liquid gels composed of cellulose nanofiber and poly(vinyl alcohol). Mater. Today Commun. 2022, 31, 103495. [Google Scholar] [CrossRef]
- Dixit, A.; Bag, D.S.; Kalra, S.J.S. Synthesis of strong and stretchable double network (DN) hydrogels of PVA-borax and P(AM-co-HEMA) and study of their swelling kinetics and mechanical properties. Polymer 2017, 119, 263–273. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Koysuren, O.; Karaman, M.; Dinc, H. Preparation and characterization of polyvinyl borate/polyvinyl alcohol (PVB/PVA) blend nanofibers. J. Appl. Polym. Sci. 2012, 124, 2736–2741. [Google Scholar] [CrossRef]
- Kanaya, T.; Ohkura, M.; Kaji, K.; Furusaka, M.; Misawa, M. Structure of Poly(Vinyl Alcohol) Gels Studied by Wide-Angle and Small-Angle Neutron-Scattering. Macromolecules 1994, 27, 5609–5615. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Huan, S.Q.; Bai, L.; Cheng, W.L.; Han, G.P. Manufacture of electrospun all-aqueous poly(vinyl alcohol)/cellulose nanocrystal composite nanofibrous mats with enhanced properties through controlling fibers arrangement and microstructure. Polymer 2016, 92, 25–35. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Li, C.; Zhang, X.; Lu, C.; Zhang, X.; Deng, Y. High performance poly (vinyl alcohol)/cellulose nanocrystals nanocomposites manufactured by injection molding. Cellulose 2014, 21, 485–494. [Google Scholar] [CrossRef]
- Feigin, L.A.; Svergun, D.I.; Taylor, G.W. Structure Analysis by Small-Angle X-ray and Neutron Scattering; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Takeno, H. Synchrotron Small-Angle X-ray Scattering and Small-Angle Neutron Scattering Studies of Nanomaterials. In X-ray and Neutron Techniques for Nanomaterials Characterization; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 717–760. [Google Scholar]
- Percus, J.K.; Yevick, G.J. Analysis of Classical Statistical Mechanics by Means of Collective Coordinates. Phys. Rev. A At. Mol. Opt. Phys. 1958, 110, 1–13. [Google Scholar] [CrossRef]
- Takeno, H.; Aoki, Y.; Kimura, K. Effects of addition of silica nanospheres on mechanical properties of clay/sodium polyacrylate hydrogels. Mater. Today Commun. 2021, 28, 102710. [Google Scholar] [CrossRef]
- Debye, P.; Bueche, A.M. Scattering by an Inhomogeneous Solid. J. Appl. Phys. 1949, 20, 518–525. [Google Scholar] [CrossRef]
- Beaucage, G. Approximations leading to a unified exponential power-law approach to small-angle scattering. J. Appl. Crystallogr. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Beaucage, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Beaucage, G.; Kammler, H.K.; Pratsinis, S.E. Particle size distributions from small-angle scattering using global scattering functions. J. Appl. Crystallogr. 2004, 37, 523–535. [Google Scholar] [CrossRef]
- Takeno, H.; Maehara, A.; Yamaguchi, D.; Koizumi, S. A Structural Study of an Organogel Investigated by Small-Angle Neutron Scattering and Synchrotron Small-Angle X-ray Scattering. J. Phys. Chem. B 2012, 116, 7739–7745. [Google Scholar] [CrossRef]
- Guth, E. Theory of Filler Reinforcement. J. Appl. Phys. 1945, 16, 20. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Ren, N.; Matta, M.E.; Martinez, H.; Walton, K.L.; Munro, J.C.; Schneiderman, D.K.; Hillmyer, M.A. Filler-Reinforced Elastomers Based on Functional Polyolefin Prepolymers. Ind. Eng. Chem. Res. 2016, 55, 6106–6112. [Google Scholar] [CrossRef]
- Takeno, H.; Kimura, Y. Molecularweight effects on tensile properties of blend hydrogels composed of clay and polymers. Polymer 2016, 85, 47–54. [Google Scholar] [CrossRef]
- Takeno, H.; Nakamura, A. Effects of molecular mass of polymer on mechanical properties of clay/poly (ethylene oxide) blend hydrogels, and comparison between them and clay/sodium polyacrylate blend hydrogels. Colloid. Polym. Sci. 2019, 297, 641–649. [Google Scholar] [CrossRef]
- Shimizu, N.; Yatabe, K.; Nagatani, Y.; Saijyo, S.; Kosuge, T.; Igarashi, N. Software Development for Analysis of Small—Angle X ray Scattering Data. AIP Conf. Proc. 2016, 1741, 050017. [Google Scholar]
- Zhang, F.; Ilavsky, J.; Long, G.G.; Quintana, J.P.G.; Allen, A.J.; Jemian, P.R. Glassy Carbon as an Absolute Intensity Calibration Standard for Small-Angle Scattering. Metall. Mater. Trans. A 2010, 41A, 1151–1158. [Google Scholar] [CrossRef]




| Sample | Rave/Å | σsphere/Å | RHS/Å | ϕ | ξDB/Å |
|---|---|---|---|---|---|
| 3 wt% SiO2/CS/PVA/borax | 32 ± 0.1 | 0.35 ± 0.002 | 40 ± 1.5 | 0.16 ± 0.007 | 349 ± 18 |
| 7 wt% SiO2/CS/PVA/borax | 32 ± 0.007 | 0.35 ± 0.0004 | 45 ± 0.8 | 0.22 ± 0.005 | 340 ± 17 |
| Samples | E/kPa | Recovery (E)/% | εf | Recovery (εf) /% |
|---|---|---|---|---|
| CS/PVA/borax (uncut) | 25.7 ± 6.8 | - | 7.7 ± 2.4 | - |
| CS/PVA/borax (self-healed gel) | 19.4 ± 3.2 | 75 | 7.0 ± 2.0 | 91 |
| clay/CS/PVA/borax (uncut) | 37.5 ± 5.2 | - | 6.7 ± 0.3 | - |
| clay/CS/PVA/borax (self-healed gel) | 30.3 ± 2.3 | 81 | 1.1 ± 0.4 | 17 |
| SiO2/CS/PVA/borax (uncut) | 113 ± 17.9 | - | 4.2 ± 1.1 | - |
| SiO2/CS/PVA/borax (self-healed gel) | 96.9 ± 14.0 | 86 | 3.7 ± 1.2 | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeno, H.; Shikano, R.; Kikuchi, R. Mechanical Performance of Corn Starch/Poly(Vinyl Alcohol) Composite Hydrogels Reinforced by Inorganic Nanoparticles and Cellulose Nanofibers. Gels 2022, 8, 514. https://doi.org/10.3390/gels8080514
Takeno H, Shikano R, Kikuchi R. Mechanical Performance of Corn Starch/Poly(Vinyl Alcohol) Composite Hydrogels Reinforced by Inorganic Nanoparticles and Cellulose Nanofibers. Gels. 2022; 8(8):514. https://doi.org/10.3390/gels8080514
Chicago/Turabian StyleTakeno, Hiroyuki, Rina Shikano, and Rin Kikuchi. 2022. "Mechanical Performance of Corn Starch/Poly(Vinyl Alcohol) Composite Hydrogels Reinforced by Inorganic Nanoparticles and Cellulose Nanofibers" Gels 8, no. 8: 514. https://doi.org/10.3390/gels8080514