Smart Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) and Chitosan through Polyelectrolyte Complexation and Its Controlled Release Properties
Abstract
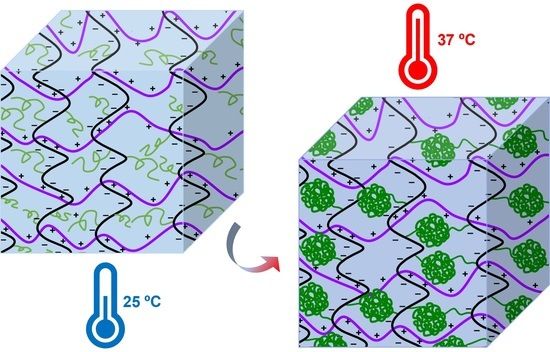
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Alginate-g-PNIPAAm
2.3. Hydrogel Formation
2.4. Rheological Studies
2.5. In Vitro Release of Rhodamine B from Hydrogels
2.6. Release of Rhodamine B from Hydrogels at Different pH
2.7. Modelling of Release Kinetics and Mechanism
3. Results and Discussions
3.1. Synthesis and Characterization of Alginate-g-PNIPAAm
3.2. PEC Hydrogel Formation
3.3. Rheological Study
3.4. In Vitro Release of Rhodamine B from Hydrogels
3.5. Effect of pH on Release Profiles
3.6. Release Kinetics and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Delv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Rel. 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Smart Hydrogel Modelling; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2009. [Google Scholar]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, S.; Islam, A.; Ghaffar, A.; Gull, N.; Hameed, A.; Bashir, A.; Jamil, T.; Hussain, T. Development of a novel pH sensitive silane crosslinked injectable hydrogel for controlled release of neomycin sulfate. Inter. J. Biol. Macromol. 2017, 97, 218–227. [Google Scholar] [CrossRef]
- Song, X.; Zhu, J.L.; Wen, Y.T.; Zhao, F.; Zhang, Z.X.; Li, J. Thermoresponsive supramolecular micellar drug delivery system based on star-linear pseudo-block polymer consisting of beta-cyclodextrin-poly(N-isopropylacrylamide) and adamantyl-poly(ethylene glycol). J. Colloid Interface Sci. 2017, 490, 372–379. [Google Scholar] [CrossRef]
- Song, X.; Wen, Y.T.; Zhu, J.L.; Zhao, F.; Zhang, Z.X.; Li, J. Thermoresponsive Delivery of Paclitaxel by beta-Cyclodextrin-Based Poly(N-isopropylacrylamide) Star Polymer via Inclusion Complexation. Biomacromolecules 2016, 17, 3957–3963. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Ni, X.P.; Li, J. Cationic brush-like terpolymer with pH responsive thickening behavior in a surfactant system. Polym. Int. 2014, 63, 1584–1592. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.X.; Zhu, J.L.; Wen, Y.T.; Zhao, F.; Lei, L.J.; Nhan, P.T.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef]
- Zhu, J.L.; Yu, S.W.K.; Chow, P.K.H.; Tong, Y.W.; Li, J. Controlling injectability and in vivo stability of thermogelling copolymers for delivery of yttrium-90 through intra-tumoral injection for potential brachytherapy. Biomaterials 2018, 180, 163–172. [Google Scholar] [CrossRef]
- Wen, Y.; Mensah, N.N.; Song, X.; Zhu, J.; Tan, W.S.; Chen, X.; Li, J. A hydrogel with supramolecular surface functionalization for cancer cell capture and multicellular spheroid growth and release. Chem. Commun. 2022, 58, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mensah, N.N.; Wen, Y.T.; Zhu, J.L.; Zhang, Z.X.; Tan, W.S.; Chen, X.W.; Li, J. beta-Cyclodextrin-Polyacrylamide Hydrogel for Removal of Organic Micropollutants from Water. Molecules 2021, 26, 5031. [Google Scholar] [CrossRef] [PubMed]
- Samchenko, Y.; Ulberg, Z.; Korotych, O. Multipurpose smart hydrogel systems. Adv. Colloid Interface Sci. 2011, 168, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Hoffman, A.S. Deswelling Characteristics of Poly(N-isopropylacrylamide) Hydrogel. J. Appl. Polym. Sci. 1994, 52, 85–89. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Huffman, A.S.; Afrassiabi, A.; Dong, L.C. Thermally Reversible Hydrogels: II. Delivery and Selective Removal of Substances from Aqueous Solutions. J. Control. Release 1986, 4, 213–222. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Liu, K.L.; Li, J. A Thermoresponsive Hydrogel Formed from a Star–Star Supramolecular Architecture. Angew. Chem. Int. Ed. 2013, 52, 6180–6184. [Google Scholar] [CrossRef]
- Diao, B.; Zhang, Z.; Zhu, J.; Li, J. Biomass-Based Thermogelling Copolymers Consisting of Lignin and Grafted Poly(N-isopropylacrylamide), Poly(ethylene glycol), and Poly(propylene glycol). RSC Adv. 2014, 4, 42996–43003. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Chen, Z.; Song, X.; Soh, W.W.M.; Wen, Y.; Zhu, J.; Zhang, M.; Li, J. In Situ Synthesis of Magnetic Poly(DMAEAB-co-NIPAm)@Fe3O4 Composite Hydrogel for Removal of Dye from Water. Gels 2021, 7, 201. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft Copolymers That Exhibit Temperature-Induced Phase Transitions over a Wide Range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-X.; Liu, X.; Xu, F.J.; Loh, X.J.; Kang, E.-T.; Neoh, K.-G.; Li, J. Pseudo-Block Copolymer Based on Star-Shaped Poly(N-isopropylacrylamide) with a β-Cyclodextrin Core and Guest-Bearing PEG: Controlling Thermoresponsivity through Supramolecular Self-Assembly. Macromolecules 2008, 41, 5967–5970. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Liu, K.L.; Li, J. Self-Assembly and Micellization of a Dual Thermoresponsive Supramolecular Pseudo-Block Copolymer. Macromolecules 2011, 44, 1182–1193. [Google Scholar] [CrossRef]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions. Langmuir 2007, 23, 196–201. [Google Scholar] [CrossRef]
- Fitzpatrick, S.D.; Jafar Mazumder, M.A.; Lasowski, F.; Fitzpatrick, L.E.; Sheardown, H. PNIPAAm-Grafted-Collagen as an Injectable, In Situ Gelling, Bioactive Cell Delivery Scaffold. Biomacromolecules 2010, 11, 2261–2267. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Lee, J.; Park, S.-Y.; Kwark, Y.-J.; Lee, K.Y. Doxorubicin-Loaded Alginate-g-Poly(N-isopropylacrylamide) Micelles for Cancer Imaging and Therapy. ACS Appl. Mater. Interfaces 2014, 6, 22069–22077. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.T.; Song, X.; Zhu, J.L.; Li, J. A smart thermoresponsive adsorption system for efficient copper ion removal based on alginate-g-poly(N-isopropylacrylamide) graft copolymer. Carbohydr. Polym. 2019, 219, 280–289. [Google Scholar] [CrossRef]
- Işıklan, N.; Küçükbalcı, G. Microwave-Induced Synthesis of Alginate-Graft-Poly(N-isopropylacrylamide) and Drug Release Properties of Dual pH- and Temperature-Responsive Beads. Eur. J. Pharm. Biopharm. 2012, 82, 316–331. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/Temperature-Responsive Behaviors of Semi-IPN and Comb-Type Graft Hydrogels Composed of Alginate and Poly(N-isopropylacrylamide). Polymer 2001, 42, 6851–6857. [Google Scholar] [CrossRef]
- Lencina, M.M.S.; Ciolino, A.E.; Andreucetti, N.A.; Villar, M.A. Thermoresponsive Hydrogels Based on Alginate-g-Poly(N-isopropylacrylamide) Copolymers Obtained by Low Doses of Gamma Radiation. Eur. Polym. J. 2015, 68, 641–649. [Google Scholar] [CrossRef]
- Cheaburu, C.N.; Ciocoiu, O.-N.; Staikos, G.; Vasile, C. Thermoresponsive Sodium Alginate-g-Poly(N-isopropylacrylamide) Copolymers III. Solution Properties. J. Appl. Polym. Sci. 2013, 127, 3340–3348. [Google Scholar] [CrossRef]
- Rao, A.; Vásquez-Quitral, P.; Fernández, M.S.; Berg, J.K.; Sánchez, M.; Drechsler, M.; Neira-Carrillo, A.; Arias, J.L.; Gebauer, D.; Cölfen, H. pH-Dependent Schemes of Calcium Carbonate Formation in the Presence of Alginates. Cryst. Growth Des. 2016, 16, 1349–1359. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, X.; Chen, Y.; Guo, J.; Chen, Q.; Jiang, X. pH-Induced Self-Assembly and Capsules of Sodium Alginate. Biomacromolecules 2005, 6, 2189–2196. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Wei, H.; Zhang, Q.; Zhang, X.-Z.; Cheng, S.-X.; Zhuo, R.-X. Effect of Ions on the Aggregation Behavior of Natural Polymer Alginate. J. Phys. Chem. B 2009, 113, 14839–14843. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, W.; Li, T.; Liu, C. Improved Physical and in Vitro Digestion Stability of a Polyelectrolyte Delivery System Based on Layer-by-Layer Self-Assembly Alginate–Chitosan-Coated Nanoliposomes. J. Agric. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, K.; Bentley, W.E.; Deng, H.; Du, Y.; Payne, G.F.; Shi, X.-W. Compartmentalized Multilayer Hydrogel Formation Using a Stimulus-Responsive Self-Assembling Polysaccharide. ACS Appl. Mater. Interfaces 2014, 6, 2948–2957. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Yuan, W.; Dong, H.; Li, C.M.; Cui, X.; Yu, L.; Lu, Z.; Zhou, Q. pH-Controlled Construction of Chitosan/Alginate Multilayer Film: Characterization and Application for Antibody Immobilization. Langmuir 2007, 23, 13046–13052. [Google Scholar] [CrossRef]
- Tapia, C.; Corbalán, V.; Costa, E.; Gai, M.N.; Yazdani-Pedram, M. Study of the Release Mechanism of Diltiazem Hydrochloride from Matrices Based on Chitosan−Alginate and Chitosan−Carrageenan Mixtures. Biomacromolecules 2005, 6, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling Concept Combining Chitosan and Alginate—Proof of Principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Araiza, R.; Alcouffe, P.; Rochas, C.; Montembault, A.; David, L. Micron Range Morphology of Physical Chitosan Hydrogels. Langmuir 2010, 26, 17495–17504. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.T.; Bai, H.Z.; Zhu, J.L.; Song, X.; Tang, G.P.; Li, J. A supramolecular platform for controlling and optimizing molecular architectures of siRNA targeted delivery vehicles. Sci. Adv. 2020, 6, eabc2148. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wen, Y.T.; Song, X.; Zhu, J.L.; Li, J. Nonviral DNA Delivery System with Supramolecular PEGylation Formed by Host-Guest Pseudo-Block Copolymers. ACS Appl. Bio Mater. 2021, 4, 5057–5070. [Google Scholar] [CrossRef]
- Li, H.; Peng, E.; Zhao, F.; Li, J.; Xue, J. Supramolecular Surface Functionalization of Iron Oxide Nanoparticles with α-Cyclodextrin-Based Cationic Star Polymer for Magnetically-Enhanced Gene Delivery. Pharmaceutics 2021, 13, 1884. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.T.; Zhu, J.L.; Song, X.; Li, J. Surface Charge Switchable Polymer/DNA Nanoparticles Responsive to Tumor Extracellular pH for Tumor-Triggered Enhanced Gene Delivery. Biomacromolecules 2020, 21, 1136–1148. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, H.; Li, J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials 2014, 35, 1050–1062. [Google Scholar] [CrossRef]
- Wen, Y.T.; Zhang, Z.X.; Li, J. Highly Efficient Multifunctional Supramolecular Gene Carrier System Self-Assembled from Redox-Sensitive and Zwitterionic Polymer Blocks. Adv. Funct. Mater. 2014, 24, 3874–3884. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Zhang, Z.; Liu, K.L.; Li, J. Supramolecular Anchoring of DNA Polyplexes in Cyclodextrin-Based Polypseudorotaxane Hydrogels for Sustained Gene Delivery. Biomacromolecules 2012, 13, 3162–3172. [Google Scholar] [CrossRef]
- Bruschi, M.L. Preface. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. vii–viii. [Google Scholar] [CrossRef]
- Leal, D.; De Borggraeve, W.; Encinas, M.V.; Matsuhiro, B.; Müller, R. Preparation and Characterization of Hydrogels Based on Homopolymeric Fractions of Sodium Alginate and PNIPAAm. Carbohydr. Polym. 2013, 92, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; She, Z.; Wang, M.; Fang, Z.; Liu, Y.; Feng, Q. Thermo-Sensitive Alginate-Based Injectable Hydrogel for Tissue Engineering. Carbohydr. Polym. 2012, 87, 1515–1521. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.L. Characterization of Temperature and pH-Responsive Poly-N-isopropylacrylamide-Co-Polymer Nanoparticles for The Release of Antimicrobials. Mater. Res. Express 2014, 1, 035405. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable Hyperbranched Amphiphilic Polyurethane Multiblock Copolymers Consisting of Poly(propylene glycol), Poly(ethylene glycol), and Polycaprolactone as In Situ Thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Loh, X.J.; Goh, S.H.; Li, J. Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol). Biomaterials 2007, 28, 4113–4123. [Google Scholar] [CrossRef]







| Sample 1 | Mn of PNIPAAm 2 | PDI of PNIPAAm 3 | DSNMR 4 | PNIPAAm Content (wt%) |
|---|---|---|---|---|
| Alg-PN31-77% | 3471 | 1.16 | 19.7 | 77 |
| Alg-PN44-72% | 4970 | 1.15 | 10.3 | 72 |
| Sample Name | Sample Type | Alginate (wt%) | PNIPAAm (wt%) | Chitosan (wt%) | Total Polymer (wt%) |
|---|---|---|---|---|---|
| Alg-PN31-77% | Copolymer hydrogel | 1.7 | 5.7 | 0 | 7.4 |
| Cts/Alg-PN31-77% | PEC hydrogel | 1.4 | 4.8 | 1.2 | 7.4 |
| Alg-PN44-72% | Copolymer hydrogel | 2.1 | 5.3 | 0 | 7.4 |
| Cts/Alg-PN44-72% | PEC hydrogel | 1.7 | 4.3 | 1.4 | 7.4 |
| Alg | Alg sol | 1.7 | 0 | 0 | 1.7 |
| Cts/Alg | PEC sol | 1.7 | 0 | 1.4 | 3.1 |
| Sample | Weight Percentage of Residual Polymers (%) | ||
|---|---|---|---|
| PBS (pH 7.4) | Acetic Buffer (pH 5.0) | Borate Buffer (pH 10.0) | |
| Alg-PN31-77% | 0 | 0 | 0 |
| Cts/Alg-PN31-77% | 39.6 ± 4.8 | 23.9 ± 7.1 | 32.5 ± 4.0 |
| Alg-PN44-72% | 0 | 3.3 ± 1.1 | 0 |
| Cts/Alg-PN44-72% | 68.7 ± 19.9 | 37.1 ± 9.5 | 41.1 ± 0.6 |
| Alg | 0 | 0 | 0 |
| Cts/Alg | 49.3 ± 8.1 | 21.8 ± 4.0 | 33.1 ± 1.4 |
| Sample | PBS (pH 7.4) | Acetic Buffer (pH 5.0) | Borate Buffer (pH 10.0) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | K (min−1) | R2 | n | K (min−1) | R2 | n | K (min−1) | R2 | |
| Alg-PN31-77% | 0.709 | 2.83 | 0.999 | 0.663 | 2.81 | 0.997 | 0.613 | 4.83 | 0.999 |
| Cts/Alg-PN31-77% | 0.525 | 4.27 | 0.998 | 0.546 | 4.59 | 0.979 | 0.612 | 3.38 | 0.991 |
| Alg-PN44-72% | 0.622 | 2.32 | 0.999 | 0.653 | 1.97 | 0.996 | 0.672 | 2.50 | 0.993 |
| Cts/Alg-PN44-72% | 0.521 | 3.94 | 0.998 | 0.616 | 2.44 | 0.981 | 0.621 | 2.64 | 0.992 |
| Alg | - | - | - | - | - | - | - | - | - |
| Cts/Alg | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhu, J.; Song, X.; Wen, Y.; Li, J. Smart Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) and Chitosan through Polyelectrolyte Complexation and Its Controlled Release Properties. Gels 2022, 8, 441. https://doi.org/10.3390/gels8070441
Liu M, Zhu J, Song X, Wen Y, Li J. Smart Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) and Chitosan through Polyelectrolyte Complexation and Its Controlled Release Properties. Gels. 2022; 8(7):441. https://doi.org/10.3390/gels8070441
Chicago/Turabian StyleLiu, Min, Jingling Zhu, Xia Song, Yuting Wen, and Jun Li. 2022. "Smart Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) and Chitosan through Polyelectrolyte Complexation and Its Controlled Release Properties" Gels 8, no. 7: 441. https://doi.org/10.3390/gels8070441