Synergistic Interaction and Binding Efficiency of Tetracaine Hydrochloride (Anesthetic Drug) with Anionic Surfactants in the Presence of NaCl Solution Using Surface Tension and UV–Visible Spectroscopic Methods
Abstract
:1. Introduction
2. Result and Discussion
2.1. Interactions of Drug with the Surfactants in the Mixed Micelle
Thermodynamic Parameters for Drug–Surfactant Mixtures in the Mixed Micelle
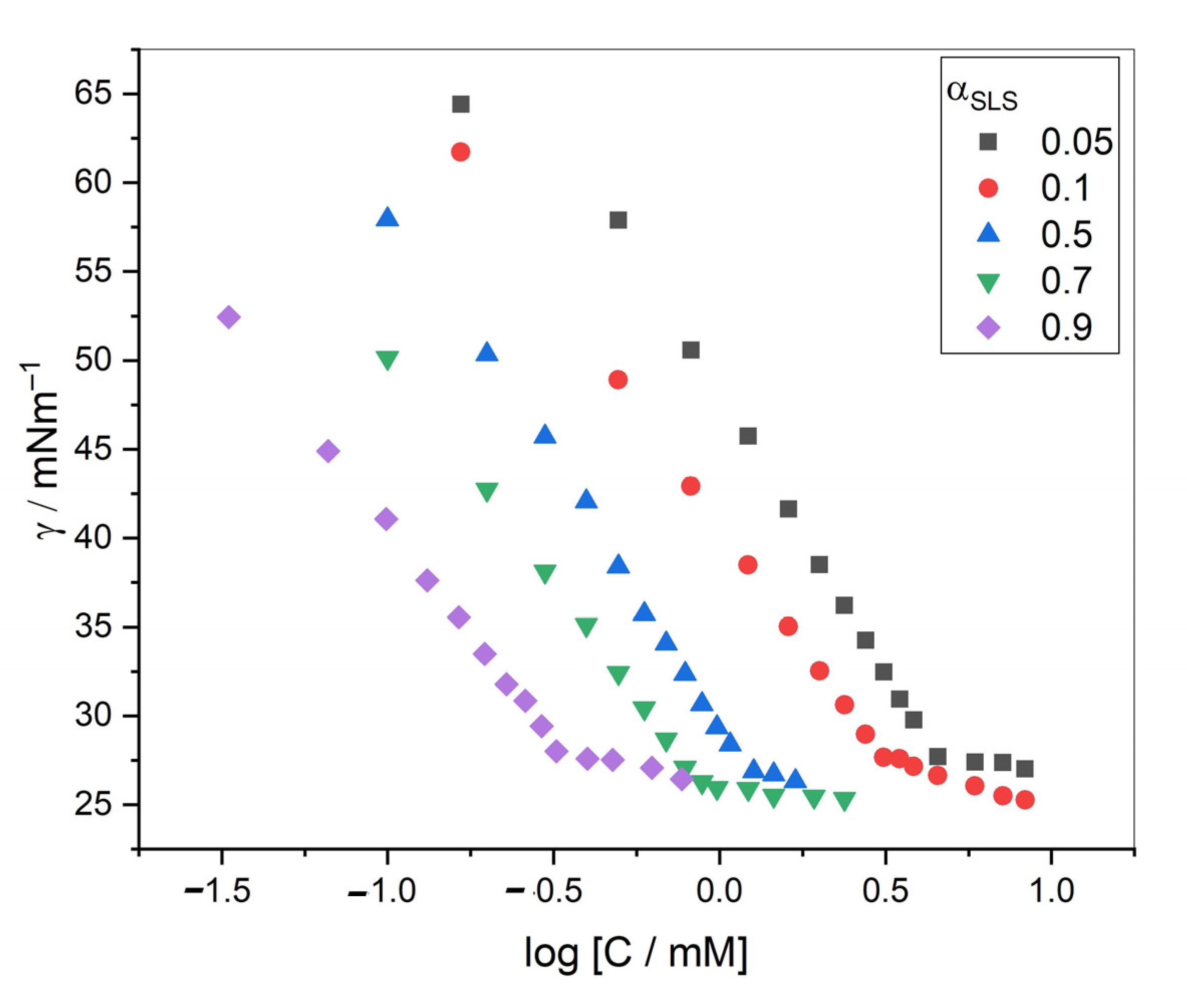
2.2. Interfacial Properties of TCH + SDS/SLS Mixed System
Thermodynamic Parameters for Drug–Surfactant Mixtures at the Surface
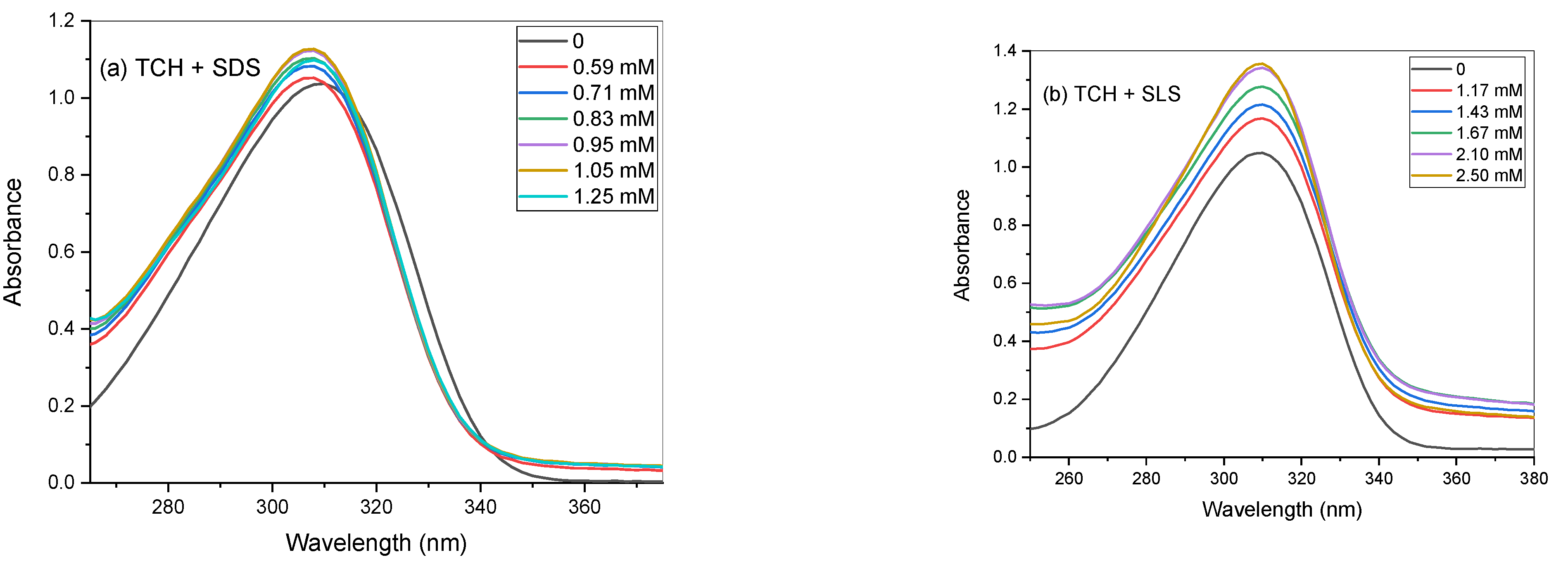
3. UV–Visible Spectroscopic Study
4. Conclusions
- The negative deviation of experimentally determined cmc values with hypothetical values confirms the nonideality of current mixtures.
- The interaction parameter at the interface and in solution was determined to be –ve, thus validating synergism between monomers of two species at the surface and in bulk.
- The higher values of the ideal mole fraction of component 1 () for both binary mixtures at all mole fractions indicate the strong ability of the drug to form of mixed micelles.
- Energetics parameters confirm the spontaneity, stability, and entropic favorability of drug–surfactant mixtures.
- The TCH with SLS had smaller binding constant values than SDS, possibly because SLS has a methylated amide nitrogen so the amide bond cannot be a hydrogen bond donor, which inhibits the intermolecular attraction between SLS and TCH at the palisade layer. Furthermore, the steric hindrance of the N-methyl group of SLS may make it difficult to tightly align the amphiphiles. All these behaviors of SLS are responsible for its smaller binding constant in comparison to SDS.
5. Experimental
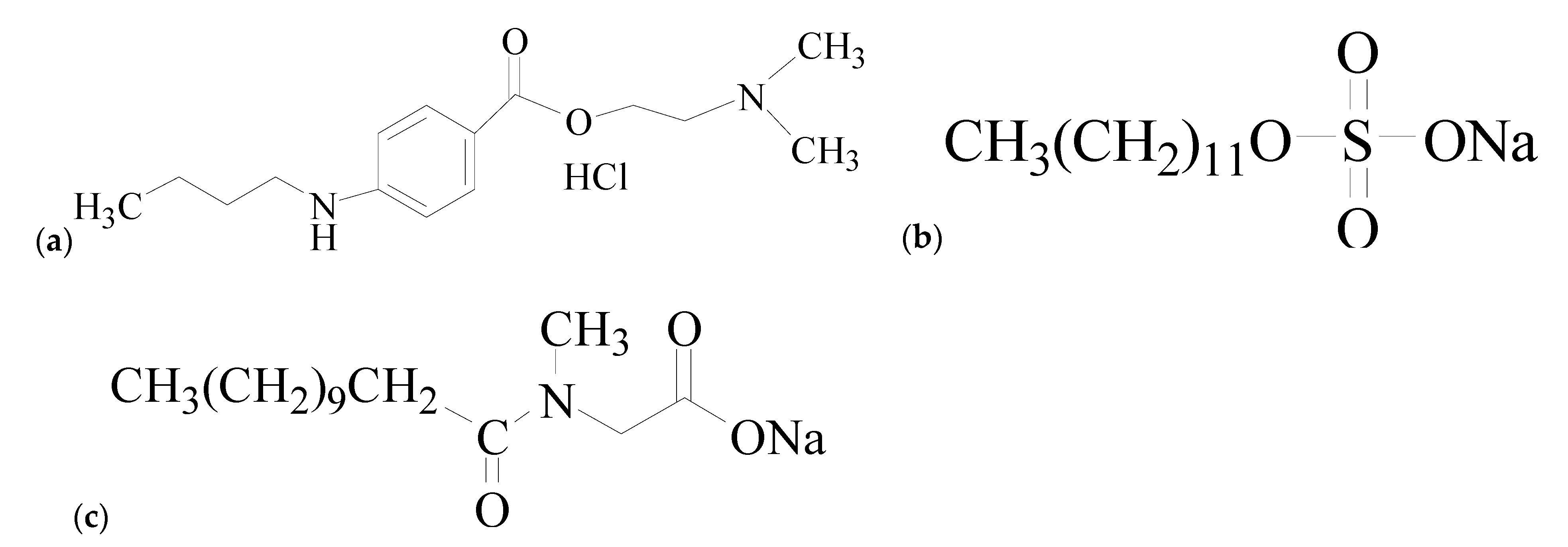
5.1. Materials
5.2. Methods
5.2.1. Surface Tension Measurements
5.2.2. UV–Vis Spectrophotometer Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P. Solubility Increases Associated with Crystalline Drug Nanoparticles: Methodologies and Significance. Mol. Pharm. 2010, 7, 1858–1870. [Google Scholar] [CrossRef]
- Ruso, J.M.; Attwood, D.; Rey, C.; Taboada, P.; Mosquera, V.; Sarmiento, F. Light Scattering and NMR Studies of the Self-Association of the Amphiphilic Molecule Propranolol Hydrochloride in Aqueous Electrolyte Solutions. J. Phys. Chem. B 1999, 103, 7092–7096. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Khan, A.; Alotaibi, M.M.; Asiri, A.M.; Rahman, M.M. Mixed Micellization, Thermodynamic and Adsorption Behavior of Tetracaine Hydrochloride in the Presence of Cationic Gemini/Conventional Surfactants. Gels 2022, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Rub, M.A.; Azum, N.; Kumar, D.; Asiri, A.M. Interaction of TX-100 and Antidepressant Imipramine Hydrochloride Drug Mixture: Surface Tension, 1H NMR, and FT-IR Investigation. Gels 2022, 8, 159. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Abdul Rub, M.; Joy, M.T.R.; Molla, M.R.; Azum, N.; Anamul Hoque, M.; Rub, M.A.; Azum, N.; Kumar, D.; Asiri, A.M.; et al. Influences of NaCl and Na2SO4 on the Micellization Behavior of the Mixture of Cetylpyridinium Chloride + Polyvinyl Pyrrolidone at Several Temperatures. Gels 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Effects of Surfactants on Gel Behavior. Am. J. Drug Deliv. 2003, 1, 77–101. [Google Scholar] [CrossRef]
- Wedel, B.; Brändel, T.; Bookhold, J.; Hellweg, T. Role of Anionic Surfactants in the Synthesis of Smart Microgels Based on Different Acrylamides. ACS Omega 2017, 2, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mitra, D.; Mitra, R.N.; Panda, A.K.; Das, P.K.; Rakshit, A.K.; Moulik, S.P. Self-Aggregation of Synthesized Novel Bolaforms and Their Mixtures with Sodium Dodecyl Sulfate (SDS) and Cetyltrimethylammonium Bromide (CTAB) in Aqueous Medium. J. Phys. Chem. B 2010, 114, 7499–7508. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Chashmi, P.; Bagheri, A. The strong synergistic interaction between surface active ionic liquid and anionic surfactant in the mixed micelle using the spectrophotometric method. J. Mol. Liq. 2018, 269, 816–823. [Google Scholar] [CrossRef]
- Mal, A.; Bag, S.; Ghosh, S.; Moulik, S.P. Physicochemistry of CTAB-SDS interacted catanionic micelle-vesicle forming system: An extended exploration. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 633–644. [Google Scholar] [CrossRef]
- Tozuka, Y.; Imono, M.; Uchiyama, H.; Takeuchi, H. A novel application of α-glucosyl hesperidin for nanoparticle formation of active pharmaceutical ingredients by dry grinding. Eur. J. Pharm. Biopharm. 2011, 79, 559–565. [Google Scholar] [CrossRef]
- Shen, S.; Ng, W.K.; Chia, L.; Dong, Y.; Tan, R.B.H. Stabilized Amorphous State of Ibuprofen by Co-Spray Drying With Mesoporous SBA-15 to Enhance Dissolution Properties. J. Pharm. Sci. 2010, 99, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Sigfridsson, K.; Lundqvist, A.J.; Strimfors, M. Particle size reduction for improvement of oral absorption of the poorly soluble drug UG558 in rats during early development. Drug Dev. Ind. Pharm. 2009, 35, 1479–1486. [Google Scholar] [CrossRef]
- Sugano, K.; Okazaki, A.; Sugimoto, S.; Tavornvipas, S.; Omura, A.; Mano, T. Solubility and Dissolution Profile Assessment in Drug Discovery. Drug Metab. Pharmacokinet. 2007, 22, 225–254. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Malheiros, S.V.P.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- YOKOYAMA, S.; FUJINO, Y.; KAWAMOTO, Y.; KANEKO, A.; FUJIE, T. Micellization of an Aqueous Solution of Piperidolate Hydrochloride in the Presence of Acetylcholine Chloride. Chem. Pharm. Bull. 1994, 42, 1351–1353. [Google Scholar] [CrossRef] [Green Version]
- Attwood, D.; Tolley, J.A. Self-association of analgesics in aqueous solution: Association models for codeine, oxycodone, ethylmorphine and pethidine. J. Pharm. Pharmacol. 2011, 32, 761–765. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Interfacial and spectroscopic behavior of phenothiazine drug/bile salt mixture in urea solution. Chem. Pap. 2021, 75, 3949–3956. [Google Scholar] [CrossRef]
- Ghosh, S.; Krishnan, A.; Das, P.K.; Ramakrishnan, S. Determination of Critical Micelle Concentration by Hyper-Rayleigh Scattering. J. Am. Chem. Soc. 2003, 125, 1602–1606. [Google Scholar] [CrossRef]
- Zhu, Q.; Huang, L.; Su, J.; Liu, S. A sensitive and visible fluorescence-turn-on probe for the CMC determination of ionic surfactants. Chem. Commun. 2014, 50, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Applied Surfactants; Wiley: Hoboken, NJ, USA, 2005; ISBN 9783527306299. [Google Scholar]
- Chiu, Y.C.; Kuo, C.Y.; Wang, C.W. Using electrophoresis to determine zeta potential of micelles and critical micelle concentration. J. Dispers. Sci. Technol. 2000, 21, 327–343. [Google Scholar] [CrossRef]
- Priev, A.; Zalipsky, S.; Cohen, R.; Barenholz, Y. Determination of Critical Micelle Concentration of Lipopolymers and Other Amphiphiles: Comparison of Sound Velocity and Fluorescent Measurements. Langmuir 2002, 18, 612–617. [Google Scholar] [CrossRef]
- Romani, A.P.; da Hora Machado, A.E.; Hioka, N.; Severino, D.; Baptista, M.S.; Codognoto, L.; Rodrigues, M.R.; de Oliveira, H.P.M. Spectrofluorimetric Determination of Second Critical Micellar Concentration of SDS and SDS/Brij 30 Systems. J. Fluoresc. 2009, 19, 327–332. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Prieto, G.; Rega, C.; Varela, L.M.; Sarmiento, F.; Mosquera, V. A Comparative Study of the Determination of the Critical Micelle Concentration by Conductivity and Dielectric Constant Measurements. Langmuir 1998, 14, 4422–4426. [Google Scholar] [CrossRef]
- Karsa, D.R. Industrial Applications of Surfactants; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Atta, A.M.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Ezzat, A.O. Demulsification of heavy crude oil using new nonionic cardanol surfactants. J. Mol. Liq. 2018, 252, 311–320. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kang, J.; Kim, D.-H. Surfactants: Recent advances and their applications. Compos. Commun. 2020, 22, 100537. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Ahmed, H. Novel cationic gemini surfactants as corrosion inhibitors for carbon steel pipelines. Corros. Sci. 2010, 52, 2897–2904. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- King, S.-Y.P.; Basista, A.M.; Torosian, G. Self-Association and Solubility Behaviors of a Novel Anticancer Agent, Brequinar Sodium. J. Pharm. Sci. 1989, 78, 95–100. [Google Scholar] [CrossRef]
- Matsuki, H.; Hashimoto, S.; Kaneshina, S.; Yamanaka, M. Surface Adsorption and Volume Behavior of Local Anesthetics. Langmuir 1994, 10, 1882–1887. [Google Scholar] [CrossRef]
- Atherton, A.D.; Barry, B.W. Photon correlation spectroscopy of surface active cationic drugs. J. Pharm. Pharmacol. 2011, 37, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; López-Fontán, J.L.; Prieto, G.; Mosquera, V.; Attwood, D. Mixed micelles of structurally related antidepressant drugs. Colloid Polym. Sci. 1997, 275, 1144–1147. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J. Phys. Org. Chem. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Abdul Rub, M.; Azum, N.; Asiri, A.M. Binary Mixtures of Sodium Salt of Ibuprofen and Selected Bile Salts: Interface, Micellar, Thermodynamic, and Spectroscopic Study. J. Chem. Eng. Data 2017, 62, 3216–3228. [Google Scholar] [CrossRef]
- Azum, N.; Naqvi, A.Z.; Rub, M.A.; Asiri, A.M. Multi-technique approach towards amphiphilic drug-surfactant interaction: A physicochemical study. J. Mol. Liq. 2017, 240, 189–195. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Bawazeer, W.A. Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems: Surface tension, fluorescence and UV–vis studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 183–192. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 2018, 31, e3730. [Google Scholar] [CrossRef]
- Khan, F.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: Micellar and thermodynamic investigation. J. Phys. Org. Chem. 2018, 31, e3812. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of antipsychotic drug with novel surfactants: Micellization and binding studies. Chin. J. Chem. Eng. 2018, 26, 566–573. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 2018, 262, 86–96. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: A tensiometry and fluorometry study. J. Chem. Thermodyn. 2018, 121, 199–210. [Google Scholar] [CrossRef]
- Azum, N.; Ahmed, A.; Rub, M.A.; Asiri, A.M.; Alamery, S.F. Investigation of aggregation behavior of ibuprofen sodium drug under the influence of gelatin protein and salt. J. Mol. Liq. 2019, 290, 111187. [Google Scholar] [CrossRef]
- Srivastava, A.; Thapa, U.; Saha, M.; Jalees, M. Aggregation behaviour of tetracaine hydrochloride with Gemini surfactants and the formation of silver nanoparticles using drug-Gemini surfactants mixture. J. Mol. Liq. 2019, 276, 399–408. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Synthesis and biological activities of local anesthetics. RSC Adv. 2019, 9, 41173–41191. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.J.; Goodwin, S.R.; Westermann-Clark, G.B.; Shah, D.O. Importance of molecular aggregation in the development of a topical local anesthetic. Langmuir 1993, 9, 105–109. [Google Scholar] [CrossRef]
- Ray, G.B.; Ghosh, S.; Moulik, S.P. Physicochemical Studies on the Interfacial and Bulk Behaviors of Sodium N-Dodecanoyl Sarcosinate (SDDS). J. Surfactants Deterg. 2009, 12, 131–143. [Google Scholar] [CrossRef]
- Umlong, I.M.; Ismail, K. Micellization behaviour of sodium dodecyl sulfate in different electrolyte media. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 8–14. [Google Scholar] [CrossRef]
- Thapa, U.; Kumar, M.; Chaudhary, R.; Singh, V.; Singh, S.; Srivastava, A. Binding behaviour of hydrophobic drug tetracaine hydrochloride used as organic counterion on ionic surfactants. J. Mol. Liq. 2021, 335, 116564. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1327. [Google Scholar] [CrossRef]
- Motomura, K.; Yamanaka, M.; Aratono, M. Thermodynamic consideration of the mixed micelle of surfactants. Colloid Polym. Sci. 1984, 262, 948–955. [Google Scholar] [CrossRef]
- Negm, N.A.; Tawfik, S.M. Studies of Monolayer and Mixed Micelle Formation of Anionic and Nonionic Surfactants in the Presence of Adenosine-5-monophosphate. J. Solut. Chem. 2012, 41, 335–350. [Google Scholar] [CrossRef]
- Ren, Z.H.; Huang, J.; Zheng, Y.C.; Lai, L.; Yu, X.R.; Chang, Y.L.; Li, J.G.; Zhang, G.H. Mixed micellization of binary mixture of amino sulfonate amphoteric surfactant with octadecyltrimethyl ammonium bromide in water/isopropanol solution: Comparison with that in aqueous solution. J. Dispers. Sci. Technol. 2019, 40, 1353–1359. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; Das, B. Formation of Mixed Micelle in an Aqueous Mixture of a Surface Active Ionic Liquid and a Conventional Surfactant: Experiment and Modeling. J. Chem. Eng. Data 2018, 63, 3784–3800. [Google Scholar] [CrossRef]
- Rosen, M.J.; Cohen, A.W.; Dahanayake, M.; Hua, X.Y. Relationship of structure to properties in surfactants. 10. Surface and thermodynamic properties of 2-dodecyloxypoly(ethenoxyethanol)s, C12H25(OC2H4)xOH, in aqueous solution. J. Phys. Chem. 1982, 86, 541–545. [Google Scholar] [CrossRef]
- Bagheri, A.; Abolhasani, A. Binary mixtures of cationic surfactants with triton X-100 and the studies of physicochemical parameters of the mixed micelles. Korean J. Chem. Eng. 2015, 32, 308–315. [Google Scholar] [CrossRef]
- Ren, Z.H.; Luo, Y.; Zheng, Y.C.; Wang, C.J.; Shi, D.P.; Li, F.X. Micellization behavior of the mixtures of amino sulfonate amphoteric surfactant and octadecyltrimethyl ammonium bromide in aqueous solution at 40 °C: A tensiometric study. J. Mater. Sci. 2015, 50, 1965–1972. [Google Scholar] [CrossRef]
- Ren, Z.H. Interacting behavior between amino sulfonate amphoteric surfactant and octylphenol polyoxyethylene ether (7) in aqueous solution and pH effect. J. Ind. Eng. Chem. 2014, 20, 3649–3657. [Google Scholar] [CrossRef]
- Zhou, Q.; Rosen, M.J. Molecular Interactions of Surfactants in Mixed Monolayers at the Air/Aqueous Solution Interface and in Mixed Micelles in Aqueous Media: The Regular Solution Approach. Langmuir 2003, 19, 4555–4562. [Google Scholar] [CrossRef]
- Rosen, M.J.; Hua, X.Y. Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interface Sci. 1982, 86, 164–172. [Google Scholar] [CrossRef]
- Ananda, K.; Yadav, O.P.; Singh, P.P. Studies on the surface and thermodynamic properties of some surfactants in aqueous and water+1,4-dioxane solutions. Colloids Surf. 1991, 55, 345–358. [Google Scholar] [CrossRef]
- Oida, T.; Nakashima, N.; Nagadome, S.; Ko, J.-S.; Oh, S.-W.; Sugihara, G. Adsorption and Micelle Formation of Mixed Surfactant Systems in Water. III. A Comparison between Cationic Gemini/Cationic and Cationic Gemini/Nonionic Combinations. J. Oleo Sci. 2003, 52, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]



| cmc (mM) | cmc* (mM) | −βRub | |||||
|---|---|---|---|---|---|---|---|
| SDS + TCH | |||||||
| 0.0 | 79.43 | - | - | - | - | - | - |
| 0.05 | 0.37 | 16.42 | 0.54 | 0.80 | 16.04 | 0.033 | 0.0095 |
| 0.1 | 0.31 | 9.16 | 0.56 | 0.90 | 15.20 | 0.055 | 0.0081 |
| 0.7 | 0.13 | 1.45 | 0.65 | 0.99 | 15.72 | 0.139 | 0.0014 |
| 0.8 | 0.15 | 1.27 | 0.66 | 0.99 | 15.44 | 0.174 | 0.0011 |
| 0.9 | 0.12 | 1.13 | 0.67 | 0.99 | 17.11 | 0.156 | 0.0004 |
| 1.0 | 1.02 | - | - | - | - | - | - |
| SLS + TCH | |||||||
| 0.0 | 79.43 | - | - | - | - | - | - |
| 0.05 | 4.49 | 41.24 | 0.50 | 0.51 | 8.86 | 0.110 | 0.1078 |
| 0.1 | 3.05 | 27.85 | 0.53 | 0.68 | 9.09 | 0.139 | 0.0742 |
| 0.5 | 1.24 | 7.74 | 0.62 | 0.95 | 9.96 | 0.243 | 0.0207 |
| 0.7 | 0.79 | 5.68 | 0.64 | 0.98 | 11.81 | 0.212 | 0.0082 |
| 0.9 | 0.33 | 4.49 | 0.64 | 0.99 | 16.53 | 0.115 | 0.0012 |
| 1.0 | 4.07 | - | - | - | - | - | - |
| α1 | ||||||
|---|---|---|---|---|---|---|
| SDS + TCH | ||||||
| 0.0 | - | - | - | - | - | 16.23 |
| 0.05 | 9.87 | 11.78 | 1.71 | 6.40 | 0.54 | 29.48 |
| 0.1 | 9.27 | 11.15 | 1.69 | 6.32 | 0.57 | 29.93 |
| 0.7 | 8.90 | 10.69 | 1.61 | 6.01 | 0.56 | 32.08 |
| 0.8 | 8.54 | 10.29 | 1.58 | 5.88 | 0.57 | 31.79 |
| 0.9 | 9.36 | 11.12 | 1.57 | 5.89 | 0.53 | 32.34 |
| 1.0 | - | - | - | - | - | 27.01 |
| SLS + TCH | ||||||
| 0.0 | - | - | - | - | - | 16.23 |
| 0.05 | 5.49 | 7.33 | 1.72 | 6.17 | 0.84 | 23.34 |
| 0.1 | 5.60 | 7.44 | 1.71 | 6.16 | 0.82 | 24.30 |
| 0.5 | 5.79 | 7.56 | 1.64 | 5.93 | 0.78 | 26.54 |
| 0.7 | 6.76 | 8.52 | 1.62 | 5.92 | 0.69 | 27.66 |
| 0.9 | 9.45 | 11.26 | 1.62 | 6.07 | 0.54 | 29.77 |
| 1.0 | - | - | - | - | - | 23.59 |
| α1 | 106 Γmax (molm−2) | Amin (Å2) | Aideal (Å2) | C20 | γcmc (mNm–1) | πcmc (mNm–1) |
|---|---|---|---|---|---|---|
| SDS + TCH | ||||||
| 0.0 | 1.64 | 1.01 | - | 19.36 | 39.57 | 31.43 |
| 0.05 | 1.77 | 0.94 | 1.01 | 0.03 | 27.79 | 43.21 |
| 0.1 | 2.44 | 0.68 | 1.01 | 0.05 | 28.55 | 42.45 |
| 0.7 | 3.10 | 0.53 | 0.99 | 0.03 | 29.88 | 41.12 |
| 0.8 | 3.39 | 0.49 | 0.98 | 0.04 | 30.17 | 40.83 |
| 0.9 | 3.28 | 0.51 | 0.97 | 0.03 | 30.68 | 40.32 |
| 1.0 | 1.71 | 0.97 | - | 0.09 | 30.60 | 40.40 |
| SLS + TCH | ||||||
| 0.0 | 1.64 | 1.01 | - | 19.36 | 39.57 | 31.43 |
| 0.05 | 2.73 | 0.61 | 1.01 | 0.80 | 27.88 | 43.11 |
| 0.1 | 2.28 | 0.73 | 1.01 | 0.41 | 27.72 | 43.28 |
| 0.5 | 2.57 | 0.65 | 1.03 | 0.19 | 26.89 | 44.11 |
| 0.7 | 2.14 | 0.77 | 1.04 | 0.09 | 27.11 | 43.89 |
| 0.9 | 2.01 | 0.83 | 1.05 | 0.04 | 28.04 | 42.96 |
| 1.0 | 1.57 | 1.05 | - | 0.18 | 23.80 | 47.20 |
| α1 | –ΔGads (kJmol−1) | Gmin (kJmol−1) | |||||
|---|---|---|---|---|---|---|---|
| SDS + TCH | |||||||
| 0.0 | - | - | - | - | - | 35.34 | 24.07 |
| 0.05 | 0.56 | 17.66 | 0.033 | 0.004 | 10.77 | 53.89 | 15.69 |
| 0.1 | 0.59 | 14.60 | 0.091 | 0.006 | 8.71 | 47.35 | 11.71 |
| 0.7 | 0.71 | 12.28 | 0.370 | 0.002 | 6.19 | 45.33 | 9.62 |
| 0.8 | 0.74 | 11.40 | 0.486 | 0.002 | 5.32 | 43.82 | 8.88 |
| 0.9 | 0.75 | 12.83 | 0.454 | 0.001 | 5.92 | 44.61 | 9.34 |
| 1.0 | - | - | - | - | - | 50.74 | 17.97 |
| SLS + TCH | |||||||
| 0.0 | - | - | - | - | - | 34.83 | 24.07 |
| 0.05 | 0.54 | 19.05 | 0.018 | 0.004 | 11.71 | 39.16 | 10.22 |
| 0.1 | 0.57 | 16.09 | 0.051 | 0.005 | 9.77 | 43.27 | 12.14 |
| 0.5 | 0.64 | 14.52 | 0.157 | 0.002 | 8.25 | 43.71 | 10.47 |
| 0.7 | 0.67 | 13.48 | 0.251 | 0.001 | 7.26 | 48.17 | 12.67 |
| 0.9 | 0.70 | 15.10 | 0.261 | 0.001 | 7.83 | 51.15 | 13.94 |
| 1.0 | - | - | - | - | 53.58 | 15.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azum, N.; Rub, M.A.; Khan, A.; Alotaibi, M.M.; Asiri, A.M. Synergistic Interaction and Binding Efficiency of Tetracaine Hydrochloride (Anesthetic Drug) with Anionic Surfactants in the Presence of NaCl Solution Using Surface Tension and UV–Visible Spectroscopic Methods. Gels 2022, 8, 234. https://doi.org/10.3390/gels8040234
Azum N, Rub MA, Khan A, Alotaibi MM, Asiri AM. Synergistic Interaction and Binding Efficiency of Tetracaine Hydrochloride (Anesthetic Drug) with Anionic Surfactants in the Presence of NaCl Solution Using Surface Tension and UV–Visible Spectroscopic Methods. Gels. 2022; 8(4):234. https://doi.org/10.3390/gels8040234
Chicago/Turabian StyleAzum, Naved, Malik Abdul Rub, Anish Khan, Maha M. Alotaibi, and Abdullah M. Asiri. 2022. "Synergistic Interaction and Binding Efficiency of Tetracaine Hydrochloride (Anesthetic Drug) with Anionic Surfactants in the Presence of NaCl Solution Using Surface Tension and UV–Visible Spectroscopic Methods" Gels 8, no. 4: 234. https://doi.org/10.3390/gels8040234








