Development of Dressing-Type Emulsion with Hydrocolloids from Butternut Squash Seed: Effect of Additives on Emulsion Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrocolloids from Butternut Squash Seed
2.2. Dressing-Type Emulsion
2.2.1. Physicochemical Properties
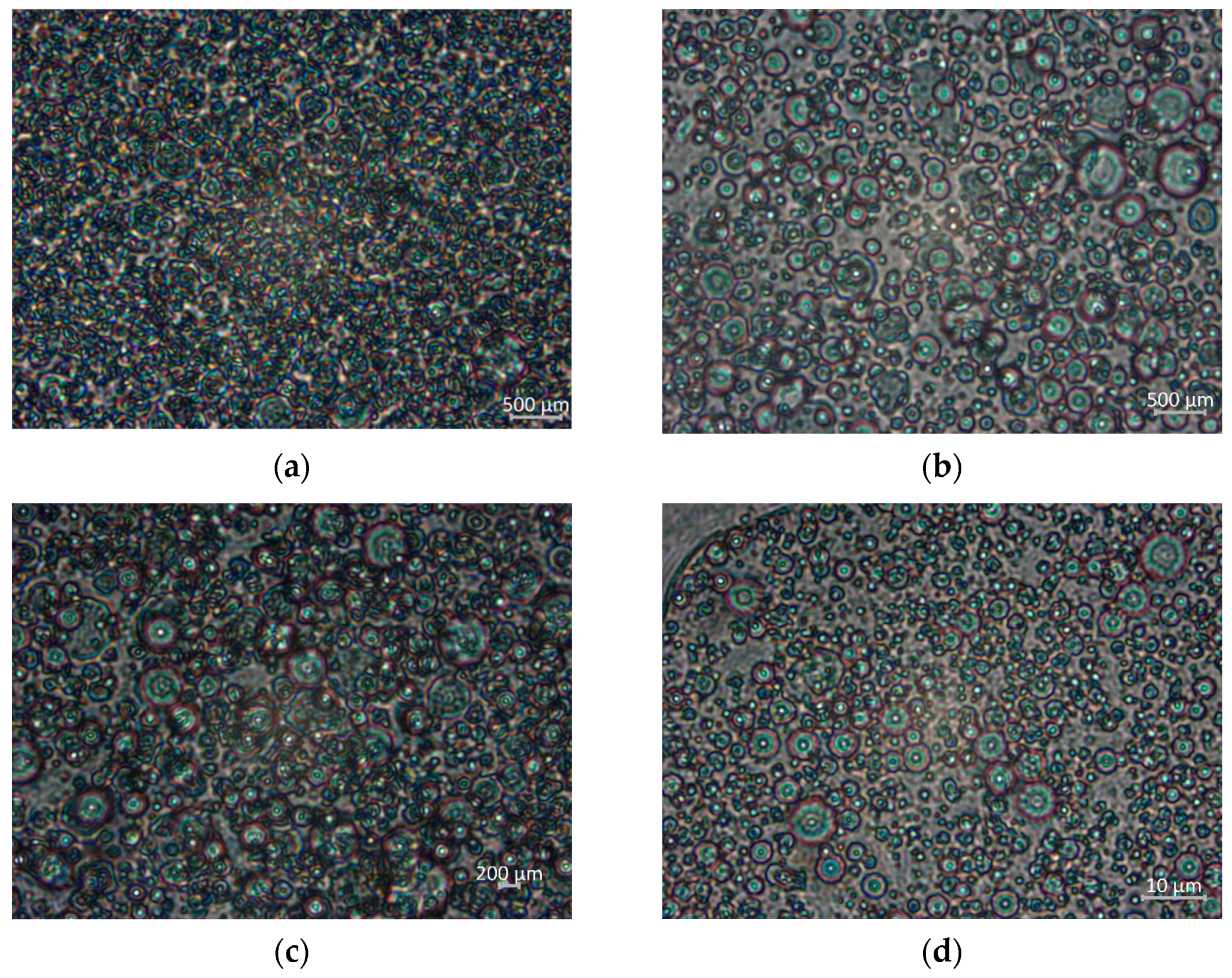
2.2.2. Microstructure Analysis
2.2.3. Rheological Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrocolloid Extraction
4.3. Formulation of Dressing Type Emulsion
4.4. Physicochemical and Proximal Composition
4.5. Rheological Properties
4.5.1. Steady Shear Analysis
4.5.2. Dynamic Oscillatory Test
4.6. Microstructural Properties
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kiosseoglou, V. Egg Yolk Protein Gels and Emulsions. Curr. Opin. Colloid Interface Sci. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Wilde, P. Interfaces: Their role in foam and emulsion behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. [Google Scholar] [CrossRef]
- Ma, L.; Barbosa-Cánovas, G. Rheological characterization of mayonnaise. Part II: Flow and viscoelastic properties at different oil and xanthan gum concentrations. J. Food Eng. 1995, 25, 409–425. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Raikos, V.; Duthie, G.; Ranawana, V. Comparing the efficiency of different food-grade emulsifiers to form and stabilise orange oil-in-water beverage emulsions: Influence of emulsifier concentration and storage time. Int. J. Food Sci. Technol. 2016, 52, 348–358. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, USA, 1998; ISBN 0849380081. [Google Scholar]
- Sainsbury, J.; Grypa, R.; Ellingworth, J.; Duodu, K.G.; De Kock, H.L. The effects of antioxidants and shelf life conditions on oxidation markers in a sunflower oil salad dressing emulsion (SOSDE). Food Chem. 2016, 213, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Drakos, A.; Kiosseoglou, V. Depletion flocculation effects in egg-based model salad dressing emulsions. Food Hydrocoll. 2008, 22, 218–224. [Google Scholar] [CrossRef]
- Gavahian, M.; Chen, Y.-M.; Khaneghah, A.M.; Barba, F.J.; Yang, B.B. In-pack sonication technique for edible emulsions: Understanding the impact of acacia gum and lecithin emulsifiers and ultrasound homogenization on salad dressing emulsions stability. Food Hydrocoll. 2018, 83, 79–87. [Google Scholar] [CrossRef]
- Diftis, N.; Biliaderis, C.; Kiosseoglou, V. Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate—dextran mixture. Food Hydrocoll. 2005, 19, 1025–1031. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Fortin, J.; Simpson, B.K.; Prasher, S.O. Rheological, physical stability, microstructural and sensory properties of salad dressings supplemented with raw and thermally treated lentil flours. J. Food Eng. 2013, 116, 862–872. [Google Scholar] [CrossRef]
- Plati, F.; Matsakidou, A.; Kiosseoglou, V.; Paraskevopoulou, A. Development of a dehydrated dressing-type emulsion with instant powder characteristics. Food Struct. 2019, 20, 100110. [Google Scholar] [CrossRef]
- Martínez, I.; Riscardo, M.A.; Franco, J.M. Effect of salt content on the rheological properties of salad dressing-type emulsions stabilized by emulsifier blends. J. Food Eng. 2007, 80, 1272–1281. [Google Scholar] [CrossRef]
- Guzey, D.; Kim, H.; McClements, D. Factors influencing the production of o/w emulsions stabilized by β-lactoglobulin—pectin membranes. Food Hydrocoll. 2004, 18, 967–975. [Google Scholar] [CrossRef]
- McArdle, R.; Hamill, R. Utilisation of hydrocolloids in processed meat systems. In Processed Meats: Improving Safety, Nutrition and Quality; Woodhead Publishing, Ltd.: Cambridge, UK, 2011; pp. 243–269. [Google Scholar]
- Bojňanská, T.; Šmitalová, J.; Vollmannová, A. Effect of the addition of hydrocolloids on the rheological and baking properties of the products with added spelt flour (Triticum spelta L.). Potravin. Slovak J. Food Sci. 2016, 10, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Balestra, F.; Petracci, M. Technofunctional Ingredients for Meat Products: Current Challenges. In Sustainable Meat Production and Processing; Academic Press: London, UK, 2018; pp. 45–68. ISBN 9780128148747. [Google Scholar]
- Quintana, S.E.; Franco, J.M.; Garcia-Zapateiro, L. Physico-chemical and bromatological characteristics of arenca and rheological properties of oil-in-water emulsions containing isolated protein. Ciência Agrotecnologia 2015, 39, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Martinez, S.; Morales-Cano, A.; Garcia Zapateiro, L. Rheological behaviour in the interaction of lecithin and guar gum for oil-in-water emulsions. Czech J. Food Sci. 2018, 36, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Orgulloso-Bautista, S.; Ortega-Toro, R.; Zapateiro, L.A.G. Design and Application of Hydrocolloids from Butternut Squash (Cucurbita moschata) Epidermis as a Food Additive in Mayonnaise-type Sauces. ACS Omega 2021, 6, 5499–5508. [Google Scholar] [CrossRef]
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra extracts as emulsifiers for acidic emulsions. Food Res. Int. 2013, 54, 1730–1737. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Wu, Y.; Eskin, N.; Cui, W.; Pokharel, B. Emulsifying properties of water soluble yellow mustard mucilage: A comparative study with gum Arabic and citrus pectin. Food Hydrocoll. 2015, 47, 191–196. [Google Scholar] [CrossRef]
- Quintana Martínez, S.E.; Torregroza Fuentes, E.E.; García Zapateiro, L.A. Food Hydrocolloids from Butternut Squash (Cucurbita moschata) Peel: Rheological Properties and Their Use in Carica papaya Jam. ACS Omega 2021, 6, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of organic acids in the fruit of different pumpkin species. Food Chem. 2014, 148, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical—Chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- Patel, S. Pumpkin (Cucurbita sp.) seeds as nutraceutic: A review on status quo and scopes. Mediterr. J. Nutr. Metab. 2013, 6, 183–189. [Google Scholar] [CrossRef]
- Nyam, K.L.; Lau, M.; Tan, C.P. Fibre from pumpkin (Cucurbita pepo L.) seeds and rinds: Physico-chemical properties, antioxidant capacity and application as bakery product ingredients. Malays. J. Nutr. 2013, 19, 99–110. [Google Scholar]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.-L.; Wang, A.T.; Inglett, G.E. Oil and Tocopherol Content and Composition of Pumpkin Seed Oil in 12 Cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of Ultrasound-Assisted Extraction and Structural Characterization of the Polysaccharide from Pumpkin (Cucurbita moschata) Seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Mazza, G.; Oomah, B.; Biliaderis, C. Optimization of an Aqueous Extraction Process for Flaxseed Gum by Response Surface Methodology. LWT Food Sci. Technol. 1994, 27, 363–369. [Google Scholar] [CrossRef]
- Somboonpanyakul, P.; Wang, Q.; Cui, W.; Barbut, S.; Jantawat, P. Malva nut gum. (Part I): Extraction and physicochemical characterization. Carbohydr. Polym. 2006, 64, 247–253. [Google Scholar] [CrossRef]
- Singthong, J.; Ningsanond, S.; Cui, S. Extraction and physicochemical characterisation of polysaccharide gum from Yanang (Tiliacora triandra) leaves. Food Chem. 2009, 114, 1301–1307. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Ibañez, M.C.; Ferrero, C. Extraction and characterization of the hydrocolloid from Prosopis flexuosa DC seeds. Food Res. Int. 2003, 36, 455–460. [Google Scholar] [CrossRef]
- Farahnaky, A.; Shanesazzadeh, E.; Mesbahi, G.; Majzoobi, M. Effect of various salts and pH condition on rheological properties of Salvia macrosiphon hydrocolloid solutions. J. Food Eng. 2013, 116, 782–788. [Google Scholar] [CrossRef]
- Vinayashree, S.; Vasu, P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021, 340, 128177. [Google Scholar] [CrossRef]
- Norton, I.T.; Norton, A.B.; Spyropoulos, F.; Le Révérend, B.J.D.; Cox, P. Rheological Control and Understanding Necessary to Formulate Healthy Everyday Foods. Pract. Food Rheol. Interpret. Approach 2010, 219–253. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Rheological characterisation of gels. J. Texture Stud. 1995, 26, 391–400. [Google Scholar] [CrossRef]
- Tabasi, S.N.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part III—Steady and dynamic shear rheology. Food Hydrocoll. 2017, 67, 243–250. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical prediction of emulsion color. Adv. Colloid Interface Sci. 2002, 97, 63–89. [Google Scholar] [CrossRef]
- Current Opinion in Colloid & Interface Science; Volume 7, Issues 5–6, Pages 253–476 (November 2002); ScienceDirect.Com by Elsevier. Available online: https://www.sciencedirect.com/journal/current-opinion-in-colloid-and-interface-science/vol/7/issue/5 (accessed on 20 August 2021).
- Hayati, I.N.; Man, Y.B.C.; Tan, C.P.; Aini, I.N. Stability and rheology of concentrated O/W emulsions based on soybean oil/palm kernel olein blends. Food Res. Int. 2007, 40, 1051–1061. [Google Scholar] [CrossRef]
- Horozov, T.S.; Binks, B.P.; Gottschalk-Gaudig, T. Effect of electrolyte in silicone oil-in-water emulsions stabilised by fumed silica particles. Phys. Chem. Chem. Phys. 2007, 9, 6398–6404. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ak, M. Dynamic oscillatory shear testing of foods—Selected applications. Trends Food Sci. Technol. 2000, 11, 115–127. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, Z.; Wu, M.; Shen, Y.; Li, G.; Ma, T. Fabrication of emulsion gel based on polymer sanxan and its potential as a sustained-release delivery system for β-carotene. Int. J. Biol. Macromol. 2020, 164, 597–605. [Google Scholar] [CrossRef]
- Bortnowska, G.; Balejko, J.; Tokarczyk, G.; Romanowska-Osuch, A.; Krzemińska, N. Effects of pregelatinized waxy maize starch on the physicochemical properties and stability of model low-fat oil-in-water food emulsions. Food Hydrocoll. 2014, 36, 229–237. [Google Scholar] [CrossRef]
- Heydari, A.; Razavi, S.M.A. Evaluating High Pressure-Treated Corn and Waxy Corn Starches as Novel Fat Replacers in Model Low-Fat O/W Emulsions: A Physical and Rheological Study. Int. J. Biol. Macromol. 2021, 184, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Heydari, A.; Razavi, S.M.A.; Hesarinejad, M.A.; Farahnaky, A. New Insights into Physical, Morphological, Thermal, and Pasting Properties of HHP-Treated Starches: Effect of Starch Type and Industry-Scale Concentration. Starch Stärke 2021, 73, 2000179. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Dolz, M.; Hernández, M.J.; Delegido, J. Oscillatory measurements for salad dressings stabilized with modified starch, xanthan gum, and locust bean gum. J. Appl. Polym. Sci. 2006, 102, 897–903. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.M.; Guo, S. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- López-Barraza, D.; Ortega-Ramos, A.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Functional Properties of Hydrocolloids from Pereskia bleo Leaves. Fluids 2021, 6, 349. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemist Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]



| Proximal Composition | pH 3 | pH 7 | pH 10 |
|---|---|---|---|
| Ys % | 4.68 ± 0.12 a | 21.17 ± 0.32 b | 31.96 ± 0.27 b |
| Moisture % | 4.42 ± 0.56 a | 3.82 ± 0.02 a | 5.04 ± 0.92 a |
| Ash % | 16.10 ± 0.85 a | 6.80 ± 0.92 b | 6.97± 0.18 b |
| Fat % | 0.96 ± 0.05 a | 6.43 ± 0.05 b | 5.83 ± 0.99 b |
| Protein % | 29.43 ± 0.17 a | 36.70 ± 0.38 b | 39.30 ± 0.21 b |
| Carbohydrate% | 50.05 ± 1.10 a | 52.68 ± 0.34 a | 52.09 ± 0.23 a |
| Code Sample | Oil % | Water % | Lecithin % | Xanthan Gum % | HBSS % |
|---|---|---|---|---|---|
| ES1 | 20 | 78.50 | 0.50 | 1.00 | 0 |
| ES2 | 20 | 78.50 | 0.50 | 0.75 | 0.25 |
| ES3 | 20 | 78.50 | 0.50 | 0.50 | 0.50 |
| ES4 | 20 | 78.50 | 0.50 | 0.25 | 0.75 |
| Sample Code | pH | °Brix | Acidity % Citric Acid | Moisture % | Ash % | Fat % | Carbohydrate % | Proteins % |
|---|---|---|---|---|---|---|---|---|
| ES1 | 6.65 ± 0.01 a | 3.01 ± 0.02 a | 0.075 ± 0.78 a | 64.39 ± 0.99 a | 1.12 ± 1.25 b | 20.03 ± 2.12 a | 13.37 ± 2.11 a | 0.39 ± 0.01 a |
| ES2 | 6.84 ± 0.03 a | 2.89 ± 0.01 a | 0.055 ± 1.58 a | 68.85 ± 1.99 b | 1.02 ± 1.90 b | 18.93 ± 2.01 a | 9.75 ± 0.02 a | 0.37 ± 0.01 a |
| ES3 | 6.83 ± 0.02 a | 2.67 ± 0.03 a | 0.053 ± 0.99 a | 70.18 ± 1.23 b | 0.59 ± 0.76 a | 18.07 ± 1.04 a | 10.66 ± 1.02 a | 0.39 ± 0.01 a |
| ES4 | 6.94 ± 0.04 a | 2.40 ± 0.01 a | 0.066 ± 1.41 a | 71.10 ± 2.45 b | 0.92 ± 0.68 b | 17.59 ± 3.55 a | 9.79 ± 1.77 a | 0.40 ± 0.01 a |
| Sample Code | L* | a* | b* | C* | ||
|---|---|---|---|---|---|---|
| ES1 | 85.38 ± 0.97 c | 1.25 ± 0.02 b | 7.48 ± 0.18 a | 28.49 ± 1.36 b | 1.40 ± 0.01 a | -- |
| ES2 | 65.57 ± 1.77 a | 2.97 ± 0.43 c | 13.10 ± 0.31 b | 16.47 ± 0.18 a | 1.51 ± 0.01 a | 4.35 ± 0.62 a |
| ES3 | 79.86 ± 4.91 b | 1.04 ± 0.02 b | 4.94 ± 0.01 a | 12.73 ± 0.01 a | 1.36 ± 0.01 a | 11.67 ± 2.18 b |
| ES4 | 81.20 ± 1.80 c | 0.34 ± 0.01 a | 5.72 ± 0.04 a | 89.30 ± 3.98 c | 1.37 ± 0.02 a | 182.66 ± 8.07 c |
| Sample Code | n | R2 | ||||
|---|---|---|---|---|---|---|
| ES1 | 2037.54 ± 9.71 d | 0.06 ± 0.00 b | 89.14 ± 1.63 b | 15.78 ± 0.10 b | 0.94 ± 0.04 a | 0.99 |
| ES2 | 548.73 ± 7.84 b | 0.02 ± 0.00 a | 71.06 ± 0.77 b | 1.96 ± 0.08 a | 0.89 ± 0.01 a | 0.99 |
| ES3 | 111.99 ± 1.20 a | 0.02 ± 0.00 a | 48.47 ± 0.82 a | 1.88 ± 0.07 a | 0.81 ± 0.01 a | 0.99 |
| ES4 | 970.31 ± 9.26 c | 0.05 ± 0.00 b | 59.73 ± 0.48 a | 2.43 ± 0.08 a | 0.94 ± 0.01 a | 0.99 |
| Sample Code | k′ (Pa) | n′ * | R2 | k″ (Pa) | n″ * | R2 | η * (Pa·s) | Tan δ |
|---|---|---|---|---|---|---|---|---|
| ES1 | 135.62 ± 1.12 a | 1.11 a | 0.97 | 25.70 ± 0.41 a | 1.09 a | 0.86 | 141.01 ± 0.01 a | 0.17 ± 0.01 a |
| ES2 | 65.78 ± 0.76 b | 1.13 a | 0.97 | 16.05 ± 0.30 b | 1.10 a | 0.86 | 69.61 ± 0.01 a | 0.22 ± 0.01 b |
| ES3 | 34.01 ± 0.44 c | 1.15 a | 0.97 | 9.92 ± 0.13 c | 1.14 a | 0.96 | 36.28 ± 0.01 a | 0.28 ± 0.01 b |
| ES4 | 10.72 ± 0.26 d | 1.21 a | 0.96 | 4.70 ± 0.02 d | 1.20 a | 0.99 | 11.89 ± 0.01 a | 0.42 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana, S.E.; Torregroza-Fuentes, E.; García Zapateiro, L.A. Development of Dressing-Type Emulsion with Hydrocolloids from Butternut Squash Seed: Effect of Additives on Emulsion Stability. Gels 2022, 8, 209. https://doi.org/10.3390/gels8040209
Quintana SE, Torregroza-Fuentes E, García Zapateiro LA. Development of Dressing-Type Emulsion with Hydrocolloids from Butternut Squash Seed: Effect of Additives on Emulsion Stability. Gels. 2022; 8(4):209. https://doi.org/10.3390/gels8040209
Chicago/Turabian StyleQuintana, Somaris E., Edilbert Torregroza-Fuentes, and Luis A. García Zapateiro. 2022. "Development of Dressing-Type Emulsion with Hydrocolloids from Butternut Squash Seed: Effect of Additives on Emulsion Stability" Gels 8, no. 4: 209. https://doi.org/10.3390/gels8040209







