Supramolecular Thixotropic Ionogel Electrolyte for Sodium Batteries
Abstract
:1. Introduction
2. Results and Discussion
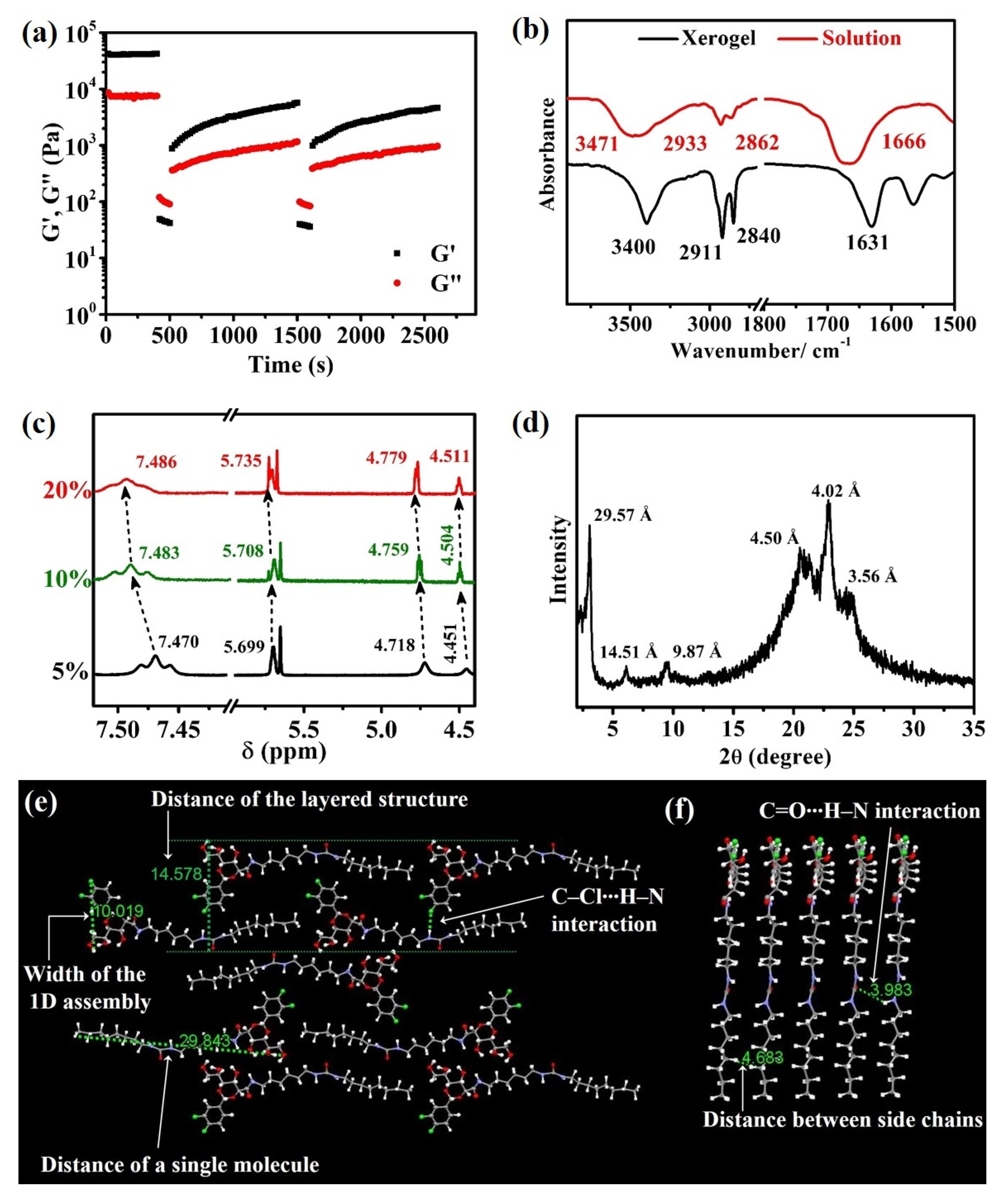
2.1. Preparation of the B8-BMPTFSI Ionogel and Insights into the Mechanism of Self-Assembly and Thixotropy
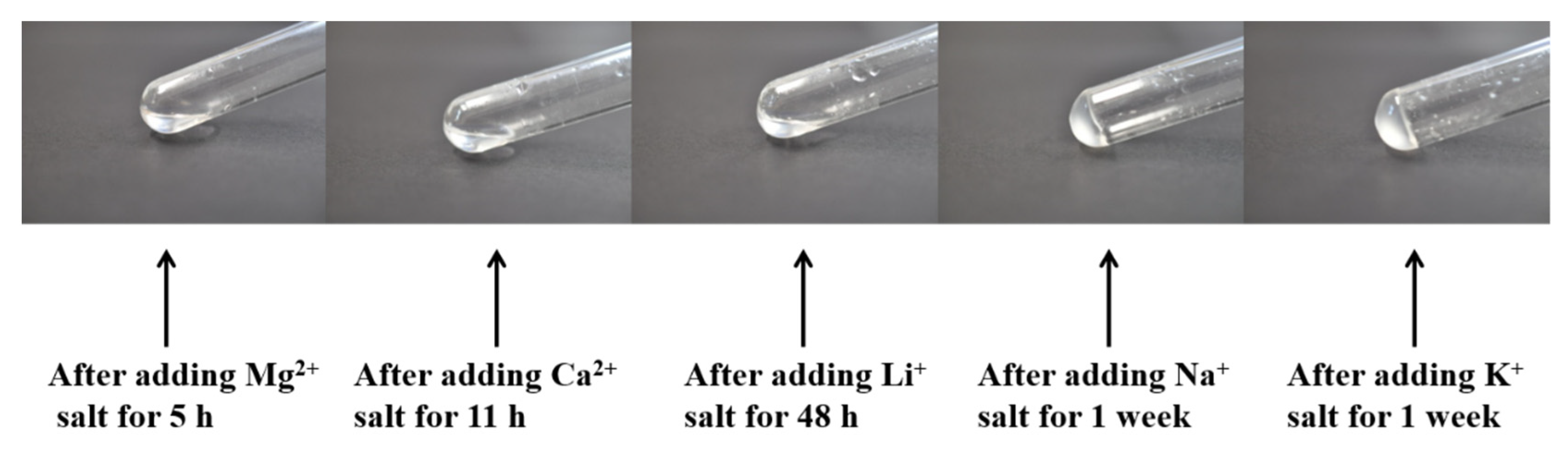
2.2. Influence of Cations on the B8-BMPTFSI Ionogel
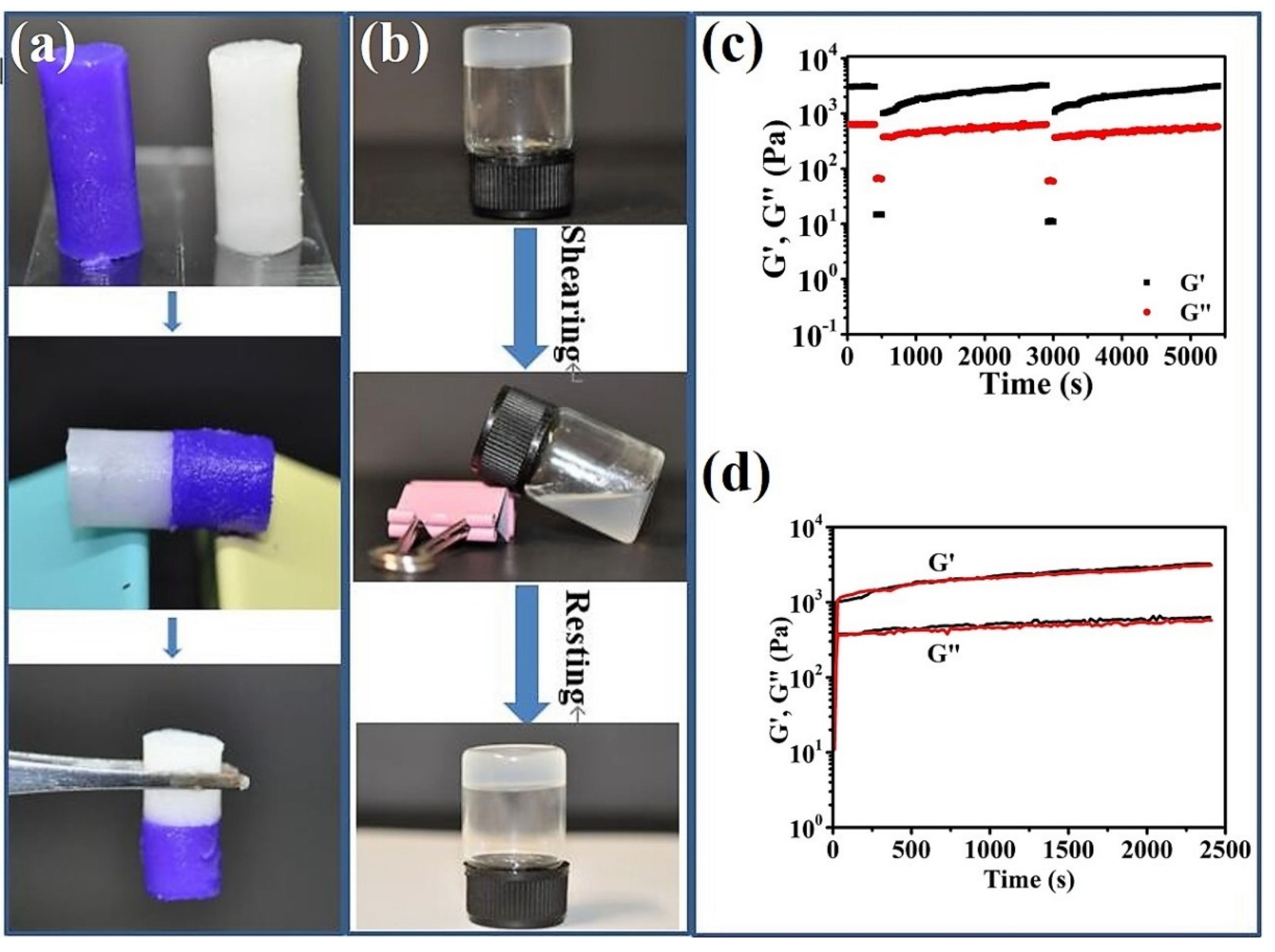
2.3. Practical Performance of the B8-BMPTFSI-NaTFSI Ionogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. Chemical Characterization
4.4. Instrumentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, P.P.; McShane, E.J.; Colclasure, A.M.; Balsara, N.; Brown, D.E.; Cao, C.T.; Chen, B.R.; Chinnam, P.R.; Cui, Y.; Dufek, E.J.; et al. A Review of Existing and Emerging Methods for Lithium Detection and Characterization in Li-Ion and Li-Metal Batteries. Adv. Energy Mater. 2021, 11, 2100372. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.W.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wang, B.; Li, J.X.; Wang, B.; Zhou, Y.; Wang, D.L.; Kliu, H.K.; Dou, S.X. Prelithiation: A Crucial Strategy for Boosting the Practical Application of Next-Generation Lithium Ion Battery. ACS Nano 2021, 15, 2197–2218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.X.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.Y.; Xu, S.M.; Sun, W.; He, Y.H. On the Sustainability of Lithium Ion Battery Industry-A Review and Perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Wang, Y.Q.; An, N.; Wen, L.; Wang, L.; Jiang, X.T.; Hou, F.; Yin, Y.X.; Liang, J. Recent Progress on the Recycling Technology of Li-Ion Batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.; Hua, H.; Liu, X.; Wu, H.; Yuan, Z. Assessment of End-of-Life Electric Vehicle Batteries in China: Future Scenarios and Economic Benefits. Waste Manag. 2021, 135, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente Crespo, M.; Van Ginkel González, M.; Talens Peiró, L. Prospects on End of Life Electric Vehicle Batteries Through 2050 in Catalonia. Resour. Conserv. Recycl. 2022, 180, 106133. [Google Scholar] [CrossRef]
- Shafique, M.; Rafiq, M.; Azam, A.; Luo, X. Material Flow Analysis for End-of-Life Lithium-Ion Batteries from Battery Electric Vehicles in the USA and China. Resour. Conserv. Recycl. 2022, 178, 106061. [Google Scholar] [CrossRef]
- Choi, Y.; Rhee, S.-W. Current Status and Perspectives on Recycling of End-of-Life Battery of Electric Vehicle in Korea (Republic of). Waste Manag. 2020, 106, 261–270. [Google Scholar] [CrossRef]
- Tian, Y.S.; Zeng, G.B.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.Y.; Koettgen, J.; Sun, Y.Z.; Ouyang, B.; Chen, T.N.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Yang, L.T.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of Today for Na-Based Batteries of the Future: From Materials to Cell Metrics. J. Power Sources 2021, 482, 27. [Google Scholar] [CrossRef]
- Wang, E.H.; Niu, Y.B.; Yin, Y.X.; Guo, Y.G. Manipulating Electrode/Electrolyte Interphases of Sodium-Ion Batteries: Strategies and Perspectives. ACS Mater. Lett. 2021, 3, 18–41. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries—A Review. Renew. Sust. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhao, L.Z.; Li, L.; Xie, M.; Wu, F.; Chen, R.J. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 41. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikov, M.; Kato, K.; Babu, G.; Kumar, N.; Mahankali, K.; Hohenstein, E.; Wang, H.; Satapathy, S.; Divya, K.P.; Asare, H.; et al. Nature-Derived Sodium-Ion Battery: Mechanistic Insights into Na-Ion Coordination within Sustainable Molecular Cathode Materials. ACS Appl. Energy Mater. 2019, 2, 8596–8604. [Google Scholar] [CrossRef]
- Syali, M.S.; Kumar, D.; Mishra, K.; Kanchan, D.K. Recent Advances in Electrolytes for Room-Temperature Sodium-Sulfur Batteries: A Review. Energy Storage Mater. 2020, 31, 352–372. [Google Scholar] [CrossRef]
- Li, K.K.; Zhang, J.; Lin, D.M.; Wang, D.W.; Li, B.H.; Lv, W.; Sun, S.; He, Y.B.; Kang, F.Y.; Yang, Q.H.; et al. Evolution of The Electrochemical Interface in Sodium Ion Batteries with Ether Electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xin, S.; Mai, L.Q.; You, Y. Materials Design for High-Safety Sodium-Ion Battery. Adv. Energy Mater. 2021, 11, 17. [Google Scholar] [CrossRef]
- Ren, W.H.; Ding, C.F.; Fu, X.W.; Huang, Y. Advanced Gel Polymer Electrolytes for Safe and Durable Lithium Metal Batteries: Challenges, Strategies, and Perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Fan, L.Q.; Tu, Q.M.; Geng, C.L.; Wang, Y.L.; Sun, S.J.; Huang, Y.F.; Wu, J.H. Improved Redox-Active Ionic Liquid-Based Ionogel Electrolyte by Introducing Carbon Nanotubes for Application in All-Solid-State Supercapacitors. Int. J. Hygrogen Energy 2020, 45, 17131–17139. [Google Scholar] [CrossRef]
- Zhou, B.H.; He, D.; Hu, J.; Ye, Y.S.; Peng, H.Y.; Zhou, X.P.; Xie, X.L.; Xue, Z.G. A Flexible, Self-Healing and Highly Stretchable Polymer Electrolyte via Quadruple Hydrogen Bonding for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 11725–11733. [Google Scholar] [CrossRef]
- Shikinaka, K.; Taki, N.; Kaneda, K.; Tominaga, Y. Quasi-Solid Electrolyte: A Thixotropic Gel of Imogolite and an Ionic Liquid. Chem. Commun. 2017, 53, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.A.M.; Yoon, H.; Forsyth, M.; MacFarlane, D.R. Gelled Ionic Liquid Sodium Ion Conductors for Sodium Batteries. Electrochim. Acta 2015, 169, 376–381. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, D.O.; Kim, S.H.; Lee, J.H.; Kim, K.M.; Oh, J.; Kim, J.; Lee, M.J.; Yang, Y.S.; Lee, S.Y.; et al. Reversible Thixotropic Gel Electrolytes for Safer and Shape-Versatile Lithium-Ion Batteries. J. Power Sources 2018, 401, 126–134. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Zhang, N.; Ge, F.; Zhang, B.; Wang, X.; Song, J. Development of Self-Healing D-Gluconic Acetal-Based Supramolecular Ionogels for Potential Use as Smart Quasisolid Electrochemical Materials. ACS Appl. Mater. Inter. 2018, 10, 5871–5879. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, N.; Zhang, B.; Zhang, B.; Song, J. Multifunctional Self-Healing Ionogels from Supramolecular Assembly: Smart Conductive and Remarkable Lubricating Materials. ACS Appl. Mater. Inter. 2018, 10, 44706–44715. [Google Scholar] [CrossRef]
- Fischer, P.J.; Do, M.P.; Reich, R.M.; Nagasubramanian, A.; Srinivasan, M.; Kuhn, F.E. Synthesis and Physicochemical Characterization of Room Temperature Ionic Liquids and Their Application in Sodium Ion Batteries. Phys. Chem. Chem. Phys. 2018, 20, 29412–29422. [Google Scholar] [CrossRef]
- Wongittharom, N.; Lee, T.C.; Wang, C.H.; Wang, Y.C.; Chang, J.K. Electrochemical Performance of Na/NaFePO4 Sodium-Ion Batteries with Ionic Liquid Electrolytes. J. Mater. Chem. A 2014, 2, 5655–5661. [Google Scholar] [CrossRef]
- Hasa, I.; Passerini, S.; Hassoun, J. Characteristics of an Ionic Liquid Electrolyte for Sodium-Ion Batteries. J. Power Sources 2016, 303, 203–207. [Google Scholar] [CrossRef]
- Barnes, H.A. Thixotropy—A Review. J. Non-Newton. Fluid Mech 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, J.J.; Lin, P.; Zhang, N.X.; Han, X.Y.; Zhang, B.; Song, J. Flexible and Highly Transparent Two-Component Organogels with Enhanced Viscoelasticity for Self-Healing Materials and Room-Temperature Phase-Selective Gelation. Chem. Commun. 2016, 52, 13975–13978. [Google Scholar] [CrossRef]
- Zhang, B.H.; Chen, S.P.; Luo, H.; Zhang, B.; Wang, F.M.; Song, J. Porous Amorphous Powder form Phase-Selective Organogelator for Rapid Recovery of Leaked Aromatics and Spilled Oils. J. Hazard. Mater. 2020, 384, 8. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Lu, R.; Zhang, P.; Jia, J.; Xu, Q.; Zhang, T.; Takafuji, M.; Ihara, H. Amplifying Emission Enhancement and Proton Response in a Two-Component Gel. Langmuir 2013, 29, 417–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Deng, M.; Shang, H.; Liang, C.; Jiang, S. An Efficient Phase-Selective Gelator for Aromatic Solvents Recovery Based on a Cyanostilbene Amide Derivative. Soft Matter 2015, 11, 5095–5100. [Google Scholar] [CrossRef]
- Xia, Q.; Mao, Y.; Wu, J.; Shu, T.; Yi, T. Two-Component Organogel for Visually Detecting Nitrite Anion. J. Mater. Chem. C 2014, 2, 1854–1861. [Google Scholar] [CrossRef]
- Chen, S.P.; Fan, Y.G.; Song, J.; Xue, B.Y. The Remarkable Role of Hydrogen Bond, Halogen, and Solvent Effect on Self-Healing Supramolecular Gel. Mater. Today Chem. 2022, 23, 11. [Google Scholar] [CrossRef]
- Srivastava, B.K.; Manheri, M.K. Towards a Fragment-Based Approach in Gelator Design: Halogen Effects Leading to Thixotropic, Mouldable and Self-Healing Systems in Aryl-Triazolyl Amino Acid-Based Gelators. Chem. Commun. 2017, 53, 4485–4488. [Google Scholar] [CrossRef]
- Chen, X.L.; Shen, Y.J.; Gao, C.; Yang, J.; Sun, X.; Zhang, X.; Yang, Y.D.; Wei, G.P.; Xiang, J.F.; Sessler, J.L.; et al. Regulating the Structures of Self-Assembled Mechanically Interlocked Moleculecular Constructs via Dianion Precursor Substituent Effects. J. Am. Soc. Chem. 2020, 142, 7443–7455. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Nanda, J.; Banerjee, A. A New Aromatic Amino Acid Based Organogel for Oil Spill Recovery. J. Mater. Chem. 2012, 22, 11658–11664. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharya, S. Remarkable Role of C-I‧‧‧N Halogen Bonding in Thixotropic ‘Halo’gel Formation. Langmuir 2016, 32, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Mallia, V.A.; Weiss, R.G. Correlations between Thixotropic and Structural Properties of Molecular Gels with Crystalline Networks. Soft Matter 2016, 12, 3665–3676. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.R.A.; Smith, D.K. Shaping and Structuring Supramolecular Gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Fan, K.; Gao, T.; Ma, A.; Zhang, B.; Song, J. A Novel Multi-Stimuli Responsive Gelator Based on D-Gluconic Acetal and Its Potential Applications. Chem. Commun. 2016, 52, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.; Rowan, A.E.; Kouwer, P.H.J. Tuning Hydrogel Mechanics Using the Hofmeister Effect. Adv. Fun. Mater. 2015, 25, 6503–6510. [Google Scholar] [CrossRef]
- Kherb, J.; Flores, S.C.; Cremer, P.S. Role of Carboxylate Side Chains in the Cation Hofmeister Series. J. Phys. Chem. B 2012, 116, 7389–7397. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, Y.; Minakuchi, N.; Mizuhata, M.; Maruyama, T. Supramolecular Gelators Based on Benzenetricarboxamides for Ionic Liquids. Soft Matter 2014, 10, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Fan, K.Q.; Niu, L.B.; Li, Y.C.; Song, J. Effects of Salt on the Gelation Mechanism of a D-Sorbitol-Based Hydrogelator. J. Phys. Chem. B 2013, 117, 5989–5995. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-Assembled Hydrogels Utilizing Polymer–Nanoparticle Interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satapathy, S.; Prasad, E. Charge Transfer Modulated Self-Assembly in Poly (aryl ether) Dendron Derivatives with Improved Stability and Transport Characteristics. ACS Appl. Mater. Inter. 2016, 8, 26176–26189. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, C.; Satapathy, S.; Jana, A.; Malakar, P.; Prasad, E.; Ghosh, S. Phenothiazine-Based Oligo(p-phenylenevinylene)s: Substituents Affected Self-Assembly, Optical Properties, and Morphology-Induced Transport. Chem. Eur. J. 2018, 24, 13213–13222. [Google Scholar] [CrossRef] [PubMed]

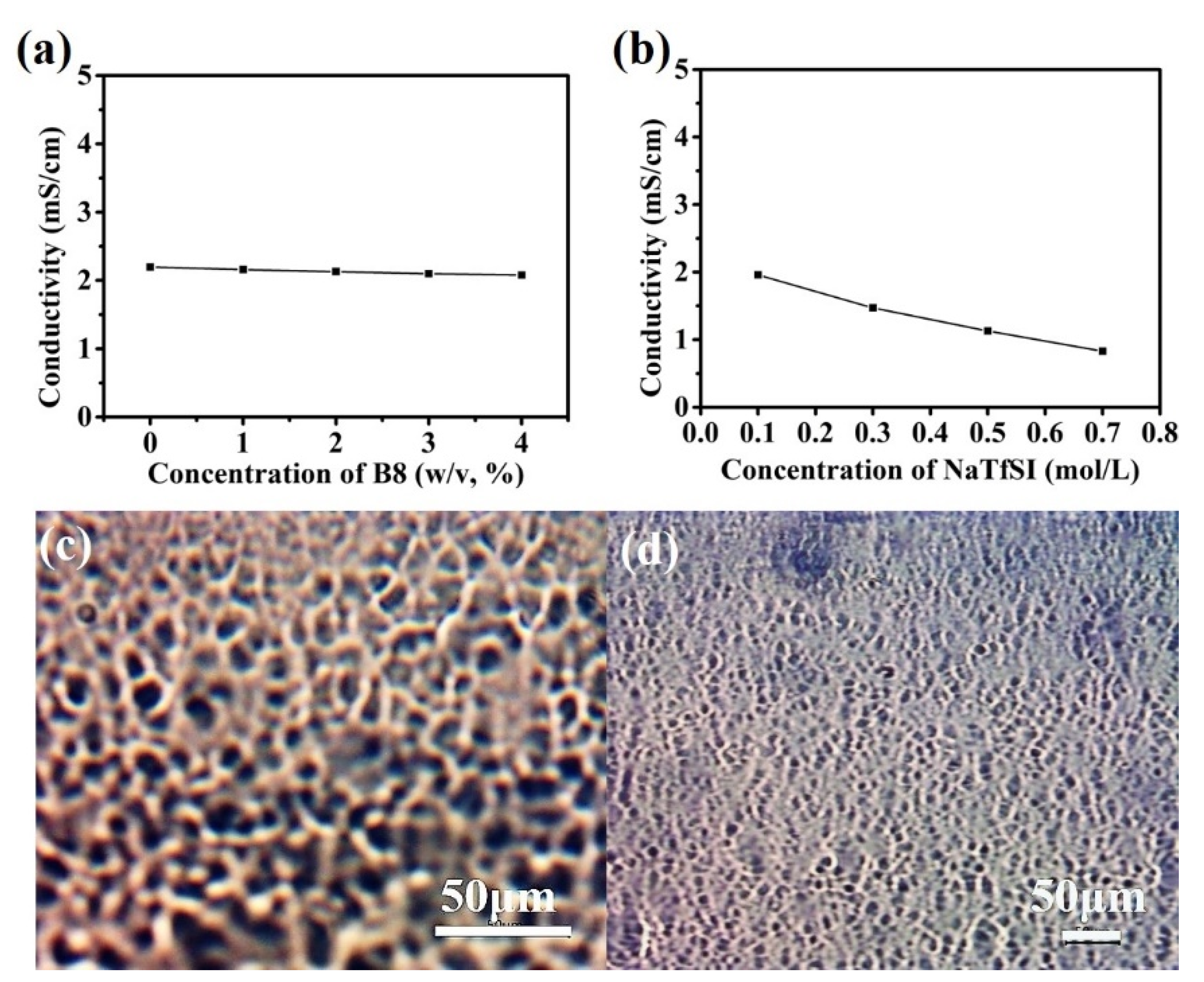

| NaTFSI Concentration | B8 | |
|---|---|---|
| CGC (w/v, %) | σ (mS/cm) | |
| 0.1M | 3 | 1.90 |
| 0.3M | 4 | 1.43 |
| 0.5M | 8 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Feng, L.; Wang, X.; Fan, Y.; Ke, Y.; Hua, L.; Li, Z.; Hou, Y.; Xue, B. Supramolecular Thixotropic Ionogel Electrolyte for Sodium Batteries. Gels 2022, 8, 193. https://doi.org/10.3390/gels8030193
Chen S, Feng L, Wang X, Fan Y, Ke Y, Hua L, Li Z, Hou Y, Xue B. Supramolecular Thixotropic Ionogel Electrolyte for Sodium Batteries. Gels. 2022; 8(3):193. https://doi.org/10.3390/gels8030193
Chicago/Turabian StyleChen, Shipeng, Li Feng, Xiaoji Wang, Yange Fan, Yubin Ke, Lin Hua, Zheng Li, Yimin Hou, and Baoyu Xue. 2022. "Supramolecular Thixotropic Ionogel Electrolyte for Sodium Batteries" Gels 8, no. 3: 193. https://doi.org/10.3390/gels8030193






