Preparation Method and Performance Evaluation of a Gel Based on AM/AMPS Copolymer
Abstract
:1. Introduction
2. Results and Discussions
2.1. Formula Optimization of Temperature and Salt Resistant Phenolic Gel
2.1.1. Effect of the AM/AMPS Copolymer Mass Fraction on the Properties of Gel
2.1.2. Effect of AMPS Content on the Properties of Gel
2.1.3. Optimization of Phenolic Cross-Linking Agent
- Optimization of crosslinking agent types
- Optimization of crosslinking agent concentration
2.1.4. Optimization of Stabilizer
- Optimization of type and mass fraction of deoxidizer
- Effect of nano-SiO2 on properties of gel
2.2. Performance Evaluation of the Prepared Gel
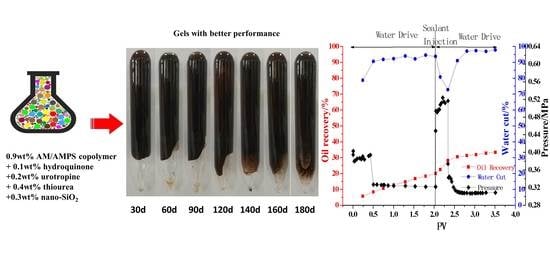
2.2.1. Evaluation of Gel in Laboratory
2.2.2. Blocking Simulation Using Two-Dimensional Plate Model
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Gel
4.2.2. Determination of Gelation Time and Strength of Gel
4.2.3. Experiment Method of Plate Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oilfield. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Cao, B.; Xie, K.; Lu, X.; Cao, W.; He, X.; Xiao, Z.; Zhang, Y.; Wang, X.; Su, C. Effect and mechanism of combined operation of profile modification and water shutoff with in-depth displacement in high-heterogeneity oil reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127673. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Pu, W.; Li, L.; Bai, B.; Han, Q.; Zhang, Y.; Tang, X. Combining preformed particle gel and curable resin-coated particles to control water production from high-temperature and high-salinity fractured producers. SPE J. 2020, 25, 938–950. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Hussein, I.A.; Sultan, A.S.; Al-Muntasheri, G.A. Gelation of emulsified polyacrylamide/polyethylenimine under high-temperature, high-salinity conditions: Rheological investigation. Ind. Eng. Chem. Res. 2018, 57, 12278–12287. [Google Scholar] [CrossRef]
- Moradi-Aragbx, A.; Bjornson, G.; Doe, P.H. Thermally Stable Gels for Near-Wellbore Permeability Contrast Corrections. SPE Adv. Technol. Ser. 1993, 1, 140–145. [Google Scholar] [CrossRef]
- Aldhaheri Munqith, W.M.; Zhang, N.; Bai, B. A Review of Field Oil-Production Response of Injuction-Well Gel Treatments. SPE Reserv. Eval. Eng. 2019, 22, 597–611. [Google Scholar] [CrossRef]
- Eriksen, O.I.; Daasvatn, K.; Vigerust, B.; Braen, T.; Olafsen, K.; Ahmed, I.; Moradi-Araghi, A.; Hamouda, A.A. Gel Formation and Thermal Stability of Gels Made from Novel Water-Soluble Polymers for Enhanced Oil Recovery Applications. In Proceedings of the Paper Presented at the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18 February 1997. [Google Scholar]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2021, 202, 108416. [Google Scholar] [CrossRef]
- Juárez, J.L.; Rodriguez, M.R.; Montes, J.; Trujillo, F.D.; Monzòn, J.; Dupuis, G.; Gaillard, N. Conformance Gel Design for High Temperature Reservoirs. In Proceedings of the Paper Presented at the SPE Europec, Virtual, Amsterdam, The Netherlands, 1–3 December 2020. [Google Scholar]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Unomah, M.; Thach, S.; Shong, R.; App, J.; Zhang, T.; Kim, D.H.; Malik, T.; Varadarajan, D. Performance of Conformance Gels Under Harsh Conditions. In Proceedings of the Paper Presented at the SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, USA, 14–18 April 2018. [Google Scholar]
- Dai, C.; Chen, W.; You, Q.; Wang, H.; Yang, Z.; He, L.; Jiao, B.; Wu, Y. A novel strengthened dispersed particle gel for enhanced oil recovery application. J. Ind. Eng. Chem. 2016, 41, 175–182. [Google Scholar] [CrossRef]
- Jokinen, M.; Györvary, E.; Rosenholm, J.B. Viscoelastic characterization of three different sol-gel derived silica gels. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 205–216. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J. Experimental investigation of the entanglement network and nonlinear viscoelastic behavior of a nano-SiO2 strengthened polymer gel. J. Mol. Liq. 2021, 339, 117288. [Google Scholar] [CrossRef]
- Ginzburg, V.V. Influence of nanoparticles on miscibility of polymer blends. A simple theory. Macromolecules 2005, 38, 2362–2367. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Fang, J.; Wang, J.; Wen, Q.; Fang, S.; He, X.; Ma, Y.; Wu, Y.; Dai, C. Research of phenolic crosslinker gel for profile control and oil displacement in high temperature and high salinity reservoirs. J. Appl. Polym. Sci. 2018, 135, 46075. [Google Scholar] [CrossRef]
- Gales, J.; Young, T.; Willhite, G.; Green, D. Equilibrium Swelling and Syneresis Properties of Xanthan Gum/Cr (III) Gels. SPE Adv. Technol. Ser. 1994, 2, 190–198. [Google Scholar] [CrossRef]
- Romero-Zeron, L.; Manalo, F.; Kantzas, A. Characterization of crosslinked gel kinetics and gel strength using NMR. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 18–20 February 2004. [Google Scholar]
- Yang, H.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.; Tang, X.; Kang, W. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- El-Karsani, K.S.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer systems for water shutoff and profile modification: A review over the last decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Zhang, T.-C.; Ge, J.-J.; Wu, H.; Guo, H.-B.; Jiao, B.-L.; Qian, Z.J.P.S. Effect of AMPS (2-acrylamido-2-methylpropane sulfonic acid) content on the properties of polymer gels. Pet. Sci. 2022, 19, 697–706. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Ge, J.; Jiang, P.; Zhu, X. Experimental research of syneresis mechanism of hpam/cr3+ gel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 96–103. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Lin, Y.; Han, S. A Novel Thermal-Resistance and Salt-Tolerance Gel with Low-Concentration Crosslinkers for Water Shutoff in Tahe Oilfield. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- Zhu, D.; Han, Y.; Zhang, J.; Li, X.; Feng, Y. Enhancing rheological properties of hydrophobically associative polyacrylamide aqueous solutions by hybriding with silica nanoparticles. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Zareie, C.; Bahramian, A.R.; Sefti, M.V.; Salehi, M.B. Network-gel strength relationship and performance improvement of polyacrylamide hydrogel using nano-silica; with regards to application in oil wells conditions. J. Mol. Liq. 2019, 278, 512–520. [Google Scholar] [CrossRef]
- Shamlooh, M.; Hamza, A.; Hussein, I.A.; Nasser, M.S.; Salehi, S. Reinforcement of polyacrylamide-co-tert-butyl acrylate base gel using nanosilica for conformance control at low and high reservoir temperatures. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 20 February 2020. [Google Scholar]
- Sydansk, R.D. A New Conformance-Improvement-Treatment Chromium (III) Gel Technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 16–21 April 1988. [Google Scholar]
- Wang, W.; Xu, Y.; Ge, J.; Guo, H.; Wu, Q.; Mao, Y. Phenolic resin gel suitable for medium-temperature and high-salinity reservoirs. J. Mol. Liq. 2022, 364, 119887. [Google Scholar] [CrossRef]











| HMTA/% | HQ/% | Resorcinol/% | Phenol/% | Gelling Time/h | Storage Modulus/Pa | Time of Dehydration Rate up to 20%/d |
|---|---|---|---|---|---|---|
| 0.1 | 0.1 | / | / | 15 | 3.3 | 70 |
| / | 0.1 | / | 360 | 3.6 | 42 | |
| 0.1 | 38 | 3.5 | 50 | |||
| 0.05 | 0.05 | / | 300 | 3.7 | 56 | |
| 0.05 | / | 0.05 | 35 | 3.5 | 46 | |
| / | 0.05 | 0.05 | 264 | 3.6 | 38 | |
| 0.08 | 0.02 | / | 96 | 3.7 | 39 | |
| 0.08 | / | 0.02 | 28 | 3.6 | 50 | |
| / | 0.08 | 0.02 | 288 | 3.6 | 33 |
| HQ/% | P-benzaldehyde/% | M-benzaldehyde/% | HMTA/% | Gelling Time/h | Storage Modulus/Pa | Time of Dehydration Rate up to 20%/d |
|---|---|---|---|---|---|---|
| 0.1 | / | / | 0.1 | 15 | 3.3 | 70 |
| 0.1 | / | / | No gel formed | / | / | |
| / | 0.1 | / | No gel formed | / | / |
| Crosslinking Agent | Thiourea /wt% | Sodium Nitrite/wt% | Sodium Thiosulfate/wt% | Gelling Time/h | Storage Modulus/Pa | Time of Dehydration Rate up to 20%/d |
|---|---|---|---|---|---|---|
| 0.1 wt% HQ + 0.2 wt% HMTA | 0.4 | / | / | 13 | 6.2 | 107 |
| / | 0.4 | / | 36 | 2.3 | 52 | |
| / | / | 0.4 | 44 | 4.7 | 58 |
| Ions | Concentration, g/L |
|---|---|
| Sodium | 73.30 |
| Calcium | 11.27 |
| Magnesium | 1.52 |
| Chloride | 137.53 |
| Bicarbonate | 0.18 |
| TDS | 223.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Preparation Method and Performance Evaluation of a Gel Based on AM/AMPS Copolymer. Gels 2022, 8, 802. https://doi.org/10.3390/gels8120802
Ran Y, Zhang G, Jiang P, Pei H. Preparation Method and Performance Evaluation of a Gel Based on AM/AMPS Copolymer. Gels. 2022; 8(12):802. https://doi.org/10.3390/gels8120802
Chicago/Turabian StyleRan, Yunling, Guicai Zhang, Ping Jiang, and Haihua Pei. 2022. "Preparation Method and Performance Evaluation of a Gel Based on AM/AMPS Copolymer" Gels 8, no. 12: 802. https://doi.org/10.3390/gels8120802