Mechanically Interlocked Hydrogel–Elastomer Strain Sensor with Robust Interface and Enhanced Water—Retention Capacity
Abstract
:1. Introduction
2. Results and Discussion
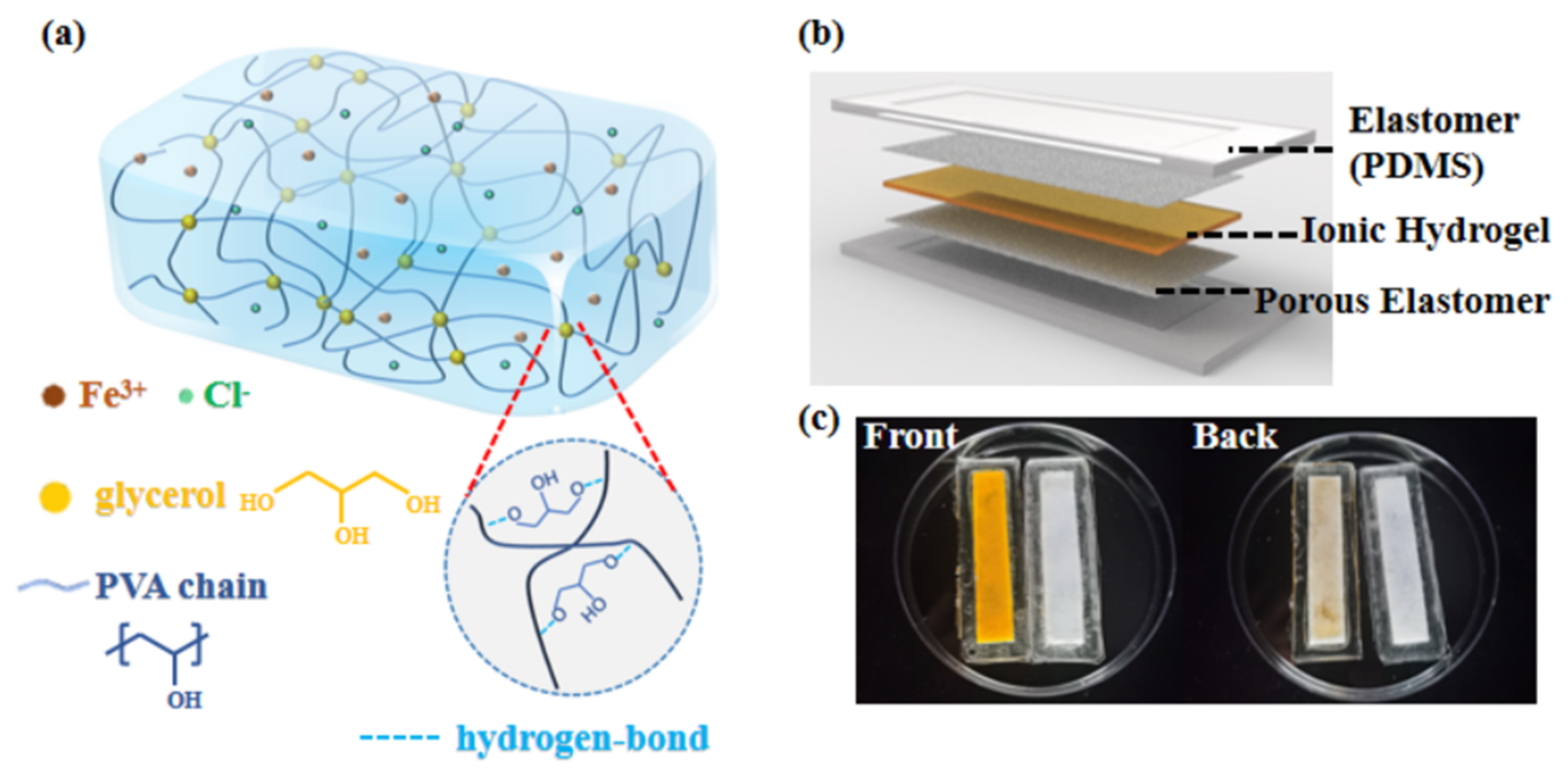
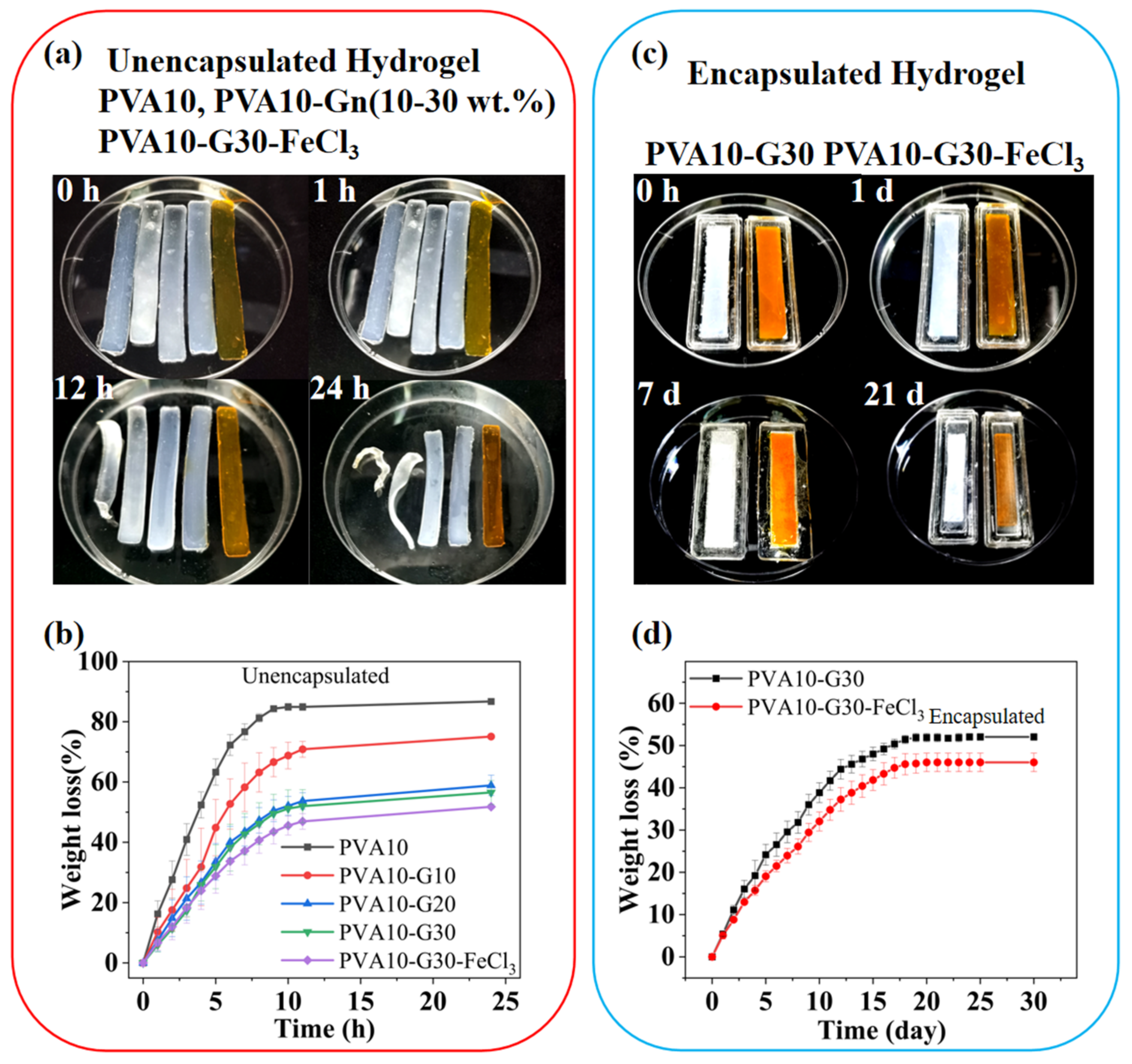
2.1. Anti-Dehydration Property of the Hydrogels
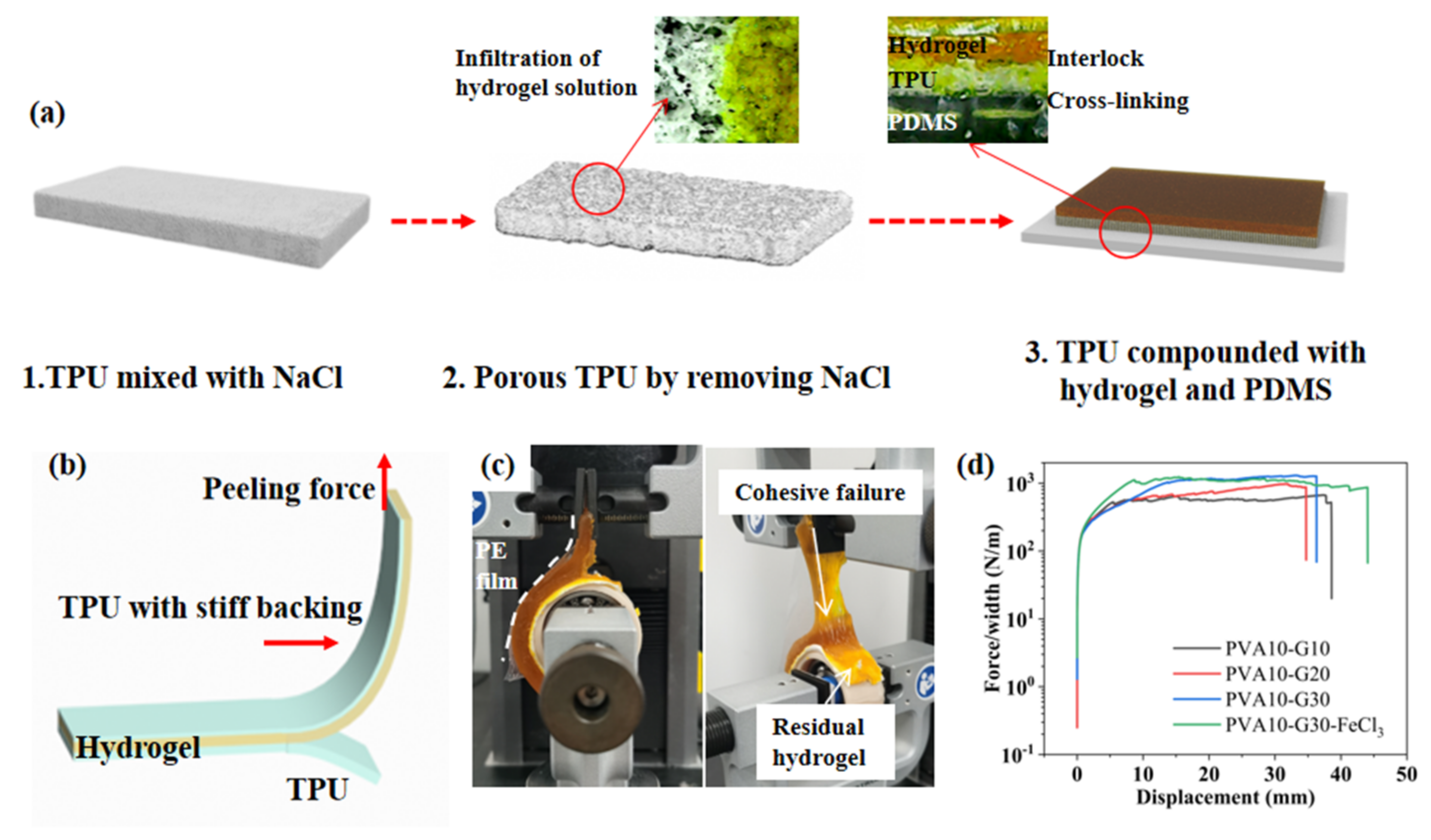
2.2. Robustness of the Hydrogel-Elastomer Hybrid
2.2.1. Interfacial Toughness of the Hydrogel-Elastomer Hybrid
2.2.2. Modulus Adaptation of the Hydrogel-Elastomer Hybrid
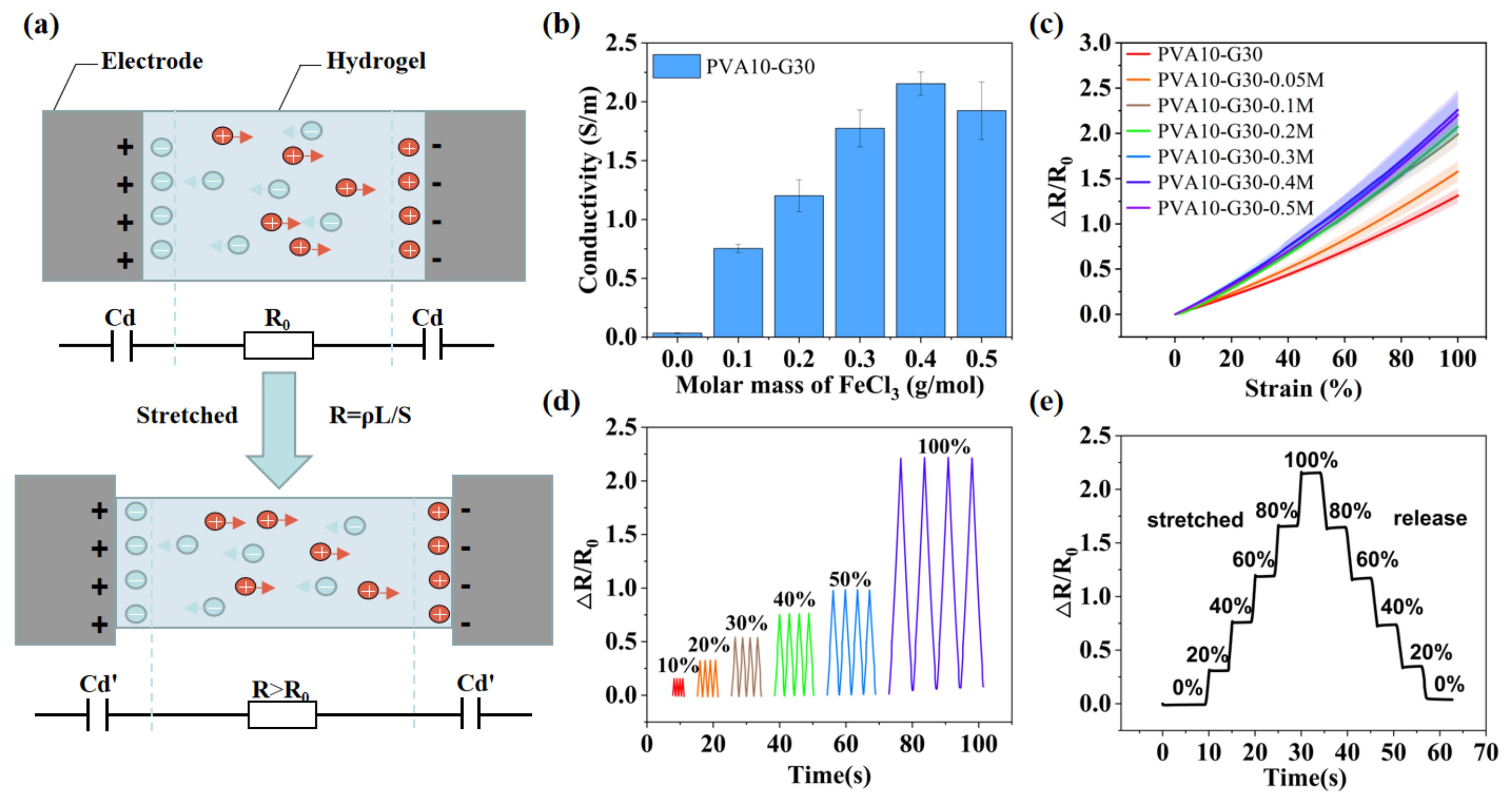
2.3. Mechanism and Properties of the IHSS
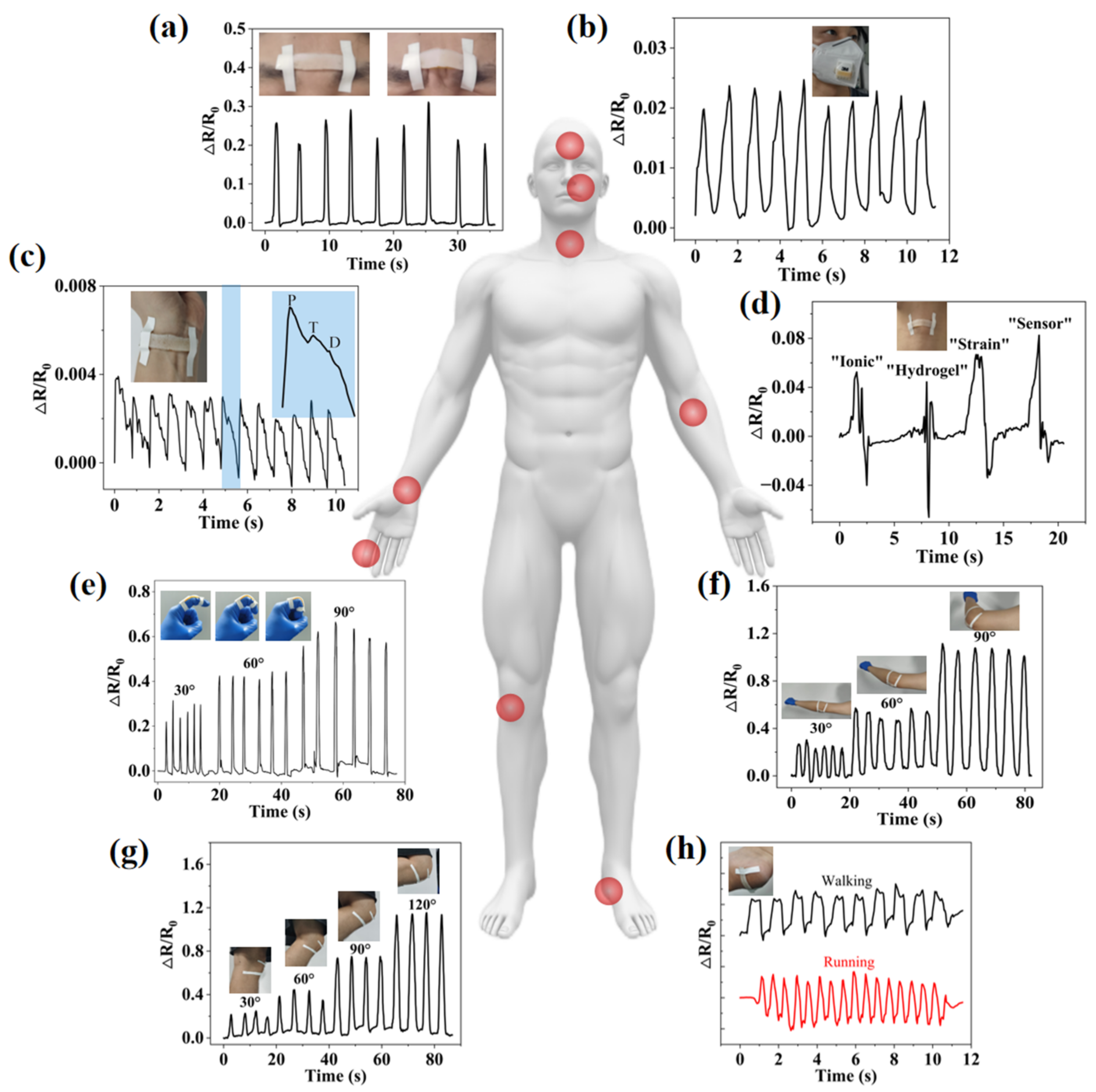
2.4. IHSS Used for Human Motion Monitoring
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Wang, L.; Xu, X.; Liu, G.; Liu, H.; Qiao, Y.; Chen, J.; Cao, S.; Cha, Q.; Wang, T. Self-Healing Materials-Based Electronic Skin: Mechanism, Development and Applications. Gels 2022, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Lee, H.-R.; Kim, C.-C.; Sun, J.-Y. Stretchable Ionics-A Promising Candidate for Upcoming Wearable Devices. Adv. Mater. 2018, 30, 1704403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Cai, X.; Yan, D.; Murtaza, G.; Meng, Z.; Qiu, L. Dual-Responsive Photonic Crystal Sensors Based on Physical Crossing-Linking SF-PNIPAM Dual-Crosslinked Hydrogel. Gels 2022, 8, 339. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Wang, L.; Wu, D. Highly Elastic and Ultratough Hybrid Ionic-Covalent Hydrogels with Tunable Structures and Mechanics. Adv. Mater. 2018, 30, 1707071. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Wang, J.; Li, T.; Zhou, X.; Gan, T.; Zhou, X. Rational fabrication of anti-freezing, non-drying tough organohydrogels by one-pot solvent displacement. Angew. Chem. 2018, 130, 6678–6681. [Google Scholar] [CrossRef]
- Dai, B.; Cui, T.; Xu, Y.; Wu, S.; Li, Y.; Wang, W.; Liu, S.; Tang, J.; Tang, L. Smart Antifreeze Hydrogels with Abundant Hydrogen Bonding for Conductive Flexible Sensors. Gels 2022, 8, 374. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Cui, X.; Wu, X.; Wang, H.; Huang, J.; Qiu, X. A Conductive, Self-Healing Hybrid Hydrogel with Excellent Water-Retention and Thermal Stability by Introducing Ethylene Glycol as a Crystallization Inhibitor. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125443. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Meng, L.; He, J.; Pan, C. Research Progress on Hydrogel-Elastomer Adhesion. Materials 2022, 15, 2548. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Dou, S.; Sun, J.; Cong, Z.; Jiang, C.; Du, C.; Pu, X.; Hu, W.; Wang, Z.L. Triboelectric-Nanogenerator-Based Soft Energy-Harvesting Skin Enabled by Toughly Bonded Elastomer/Hydrogel Hybrids. ACS Nano 2018, 12, 2818–2826. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Le, H.H.; Cui, J.; Jeon, I. Double-Hydrophobic-Coating through Quenching for Hydrogels with Strong Resistance to Both Drying and Swelling. Adv. Sci. 2020, 7, 1903145. [Google Scholar] [CrossRef]
- Feng, J.-F.; Chen, J.-L.; Guo, K.; Hou, J.-B.; Zhou, X.-L.; Huang, S.; Li, B.-J.; Zhang, S. Leeches-Inspired Hydrogel-Elastomer Integration Materials. ACS Appl. Mater. Interfaces 2018, 10, 40238–40245. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2015, 15, 190–196. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, G.; Hao, D.; Chen, L.; Liu, K.; Liu, M. Macroscopic Layered Organogel-Hydrogel Hybrids with Controllable Wetting and Swelling Performance. Adv. Funct. Mater. 2018, 28, 1800793. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-inspired double-hydrophobic-coating encapsulated hydrogels with enhanced water retention capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 2019, 48, 2946–2966. [Google Scholar] [CrossRef]
- Tian, K.; Bae, J.; Suo, Z.; Vlassak, J.J. Adhesion between Hydrophobic Elastomer and Hydrogel through Hydrophilic Modification and Interfacial Segregation. ACS Appl. Mater. Interfaces 2018, 10, 43252–43261. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Han, X.; Pan, Y.; Wang, P.; Wang, T.; Lu, T. A Universal Strategy for Tough Adhesion of Wet Soft Material. Adv. Funct. Mater. 2020, 30, 2003207. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, C.; Yang, X.; Suo, Z. Dual-primer adhesion of polymer networks of dissimilar chemistries. Extreme Mech. Lett. 2020, 38, 100756. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef]
- Stokes, R.H.; Robinson, R.A. Ionic Hydration and Activity in Electrolyte Solutions. J. Am. Chem. Soc. 1948, 70, 1870–1878. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2018, 29, 1806220. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B: Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Shafiei, M.; Langford, S.J. Nanoporous naphthalene diimide surface enhances humidity and ammonia sensing at room temperature. Sens. Actuators B Chem. 2021, 351, 130972. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Lin, Z.; Wang, X.; Wang, Z.; Sun, Z. Mechanically Interlocked Hydrogel–Elastomer Strain Sensor with Robust Interface and Enhanced Water—Retention Capacity. Gels 2022, 8, 625. https://doi.org/10.3390/gels8100625
Zhao W, Lin Z, Wang X, Wang Z, Sun Z. Mechanically Interlocked Hydrogel–Elastomer Strain Sensor with Robust Interface and Enhanced Water—Retention Capacity. Gels. 2022; 8(10):625. https://doi.org/10.3390/gels8100625
Chicago/Turabian StyleZhao, Wenyu, Zhuofan Lin, Xiaopu Wang, Ziya Wang, and Zhenglong Sun. 2022. "Mechanically Interlocked Hydrogel–Elastomer Strain Sensor with Robust Interface and Enhanced Water—Retention Capacity" Gels 8, no. 10: 625. https://doi.org/10.3390/gels8100625




