Microstructured Hyaluronic Acid Hydrogel for Tooth Germ Bioengineering
Abstract
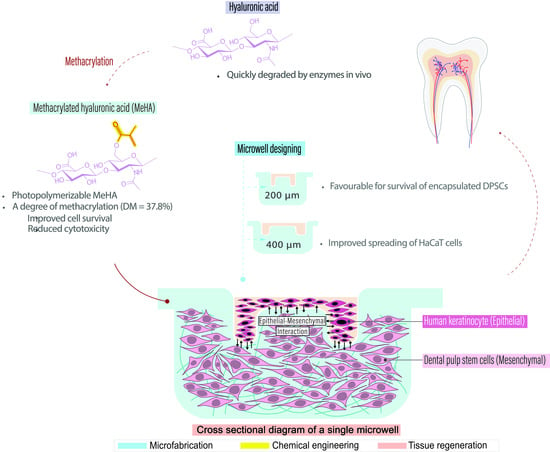
:1. Introduction
2. Results and Discussion
2.1. Nuclear Magnetic Resonance (NMR) Characterization of MeHA
2.2. Cytotoxicity Test
2.3. Fabrication of Hydrogel Microwell Array
2.4. DPSC Encapsulation within MeHA Hydrogel Microwell Array
2.5. HaCaT Cell Seeding in Hydrogel Microwell Array
2.6. Co-Culture of DPSCs and HaCaT Cells
3. Conclusions
4. Materials and Methods
4.1. MeHA Synthesis
4.2. NMR Characterization of MeHA
4.3. MeHA Prepolymer Solution Preparation
4.4. Fabrication of Polydimethylsiloxane (PDMS) Lithographical Stamp
4.5. Hydrogel Microwell Array Fabrication
4.6. Tissue Culture
4.7. Cytotoxicity Test
4.8. DPSC Encapsulation inside the Hydrogel
4.9. Cell Seeding
4.10. Co-Culture of DPSCs and HaCaT Cells
4.11. Cell Viability Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paryag, A.; Rafeek, R. Dental erosion and medical conditions: An overview of aetiology, diagnosis and management. West Indian Med. J. 2014, 63, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.J.; Saponaro, P.C. Management of edentulous patients. Dent. Clin. 2019, 63, 249–261. [Google Scholar] [CrossRef]
- Shah, R.J.; Diwan, F.J.; Diwan, M.J.; Chauhan, V.J.; Agrawal, H.S.; Patel, G.C. A study of the emotional effects of tooth loss in an edentulous Gujarati population and its association with depression. J. Indian Prosthodont. Soc. 2015, 15, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Basnet, B.B.; Adhikari, G. A questionnaire study on the impact on oral health-related quality of life by conventional rehabilitation of edentulous patient. BDJ Open 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Omiri, M.K.; Al-Masri, M.; Alhijawi, M.M.; Lynch, E. Combined implant and tooth support: An up-to-date comprehensive overview. Int. J. Dent. 2017, 2017, 6024565. [Google Scholar] [CrossRef] [PubMed]
- Al-Quran, F.A.; Al-Ghalayini, R.F.; Al-Zu’bi, B.N. Single-tooth replacement: Factors affecting different prosthetic treatment modalities. BMC Oral Health 2011, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Grusovin, M.G.; Maghaireh, H.; Worthington, H.V. Interventions for replacing missing teeth: Different times for loading dental implants. Cochrane Database Syst. Rev. 2013, 2013, CD003878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steindorff, M.M.; Lehl, H.; Winkel, A.; Stiesch, M. Innovative approaches to regenerate teeth by tissue engineering. Arch. Oral Biol. 2014, 59, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Klein, O.D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 2020, 147, dev184754. [Google Scholar] [CrossRef]
- Arakaki, M.; Ishikawa, M.; Nakamura, T.; Iwamoto, T.; Yamada, A.; Fukumoto, E.; Saito, M.; Otsu, K.; Harada, H.; Yamada, Y.; et al. Role of epithelial-stem cell interactions during dental cell differentiation. J. Biol. Chem. 2012, 287, 10590–10601. [Google Scholar] [CrossRef] [Green Version]
- Cobourne, M.T.; DiBiase, A.T. Handbook of Orthodontics; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yelick, P.C.; Sharpe, P.T. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef]
- Yu, J.; Shi, J.; Jin, Y. Current approaches and challenges in making a bio-tooth. Tissue Eng. Part B Rev. 2008, 14, 307–319. [Google Scholar] [CrossRef]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, N.; Mala, K. Regenerative endodontics as a tissue engineering approach: Past, current and future. Aust. Endod. J. 2012, 38, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gordillo, V.; Chmielewski, J. Mimicking the extracellular matrix with functionalized, metal-assembled collagen peptide scaffolds. Biomaterials 2014, 35, 7363–7373. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, E.; Tsuji, T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin. Biol. Ther. 2008, 8, 735–744. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering - a review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.A.; Chen, Y.-H.; Wong, T.Y.; Bittencourt, V.Z.; Lin, Y.-C.; Huang, L.L.H. Hyaluronan regulates cell behavior: A potential niche matrix for stem cells. Biochem. Res. Int. 2012, 2012, 346972. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Weng, L.; Gouldstone, A.; Wu, Y.; Chen, W. Mechanically strong double network photocrosslinked hydrogels from N, N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterials 2008, 29, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Baier Leach, J.; Bivens, K.A.; Patrick Jr, C.W.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Xiao, L.; Tsutsui, T. Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: Expression patterns of relevant molecules. J. Cell. Biochem. 2012, 113, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. 2016, 45, 124–131. [Google Scholar] [CrossRef]
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning 2020, 13, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluteau, G.; Luder, H.U.; De Bari, C.; Mitsiadis, T.A. Stem cells for tooth engineering. Eur. Cell Mater. 2008, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-H.; Chen, B.; Zhu, Q.-L.; Kong, H.; Li, Q.-H.; Gao, L.-N.; Xiao, M.; Chen, F.-M.; Yu, Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014, 35, 9459–9472. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tezuka, Y.; Horiuchi, M.; Hosono, K.; Iida, K.; Hatakeyama, D.; Miyaki, S.; Kunisada, T.; Shibata, T.; Tezuka, K. Characterization of dental pulp stem cells of human tooth germs. J. Dent. Res. 2008, 87, 676–681. [Google Scholar] [CrossRef]
- Wang, Y.; Preston, B.; Guan, G. Tooth bioengineering leads the next generation of dentistry. Int. J. Paediatr. Dent. 2012, 22, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Ligoudistianou, C.; Taraslia, V.; Kontakiotis, E.; Anastasiadou, E. Dental stem cells and their applications in dental tissue engineering. Open Dent. J. 2013, 7, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, L.; Du, S.; Liu, C.; Lin, X.; Chen, Y.; Zhang, Y. Induction of human keratinocytes into enamel-secreting ameloblasts. Dev. Biol. 2010, 344, 795–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudshoorn, M.H.M.; Rissmann, R.; Bouwstra, J.A.; Hennink, W.E. Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. Polymer 2007, 48, 1915–1920. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Eng, G.; Yeh, J.; Fukuda, J.; Blumling Iii, J.; Langer, R.; Burdick, J.A. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J. Biomed. Mater. Res. A 2006, 79A, 522–532. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, N.; Sreekumar, A.V. An in vitro investigation into the cytotoxicity of methyl methacrylate monomer. J. Contemp. Dent. Pract. 2012, 13, 838–841. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef]
- Xu, L.; Sheybani, N.; Yeudall, W.A.; Yang, H. The effect of photoinitiators on intracellular AKT signaling pathway in tissue engineering application. Biomater. Sci. 2015, 3, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Tan, J.J.Y.; Nguyen, D.V.; Common, J.E.; Wu, C.; Ho, P.C.L.; Kang, L. Investigating PEGDA and GelMA Microgel Models for Sustained 3D Heterotypic Dermal Papilla and Keratinocyte Co-Cultures. Int. J. Mol. Sci. 2021, 22, 2143. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Kang, L.; Qiu, Y.; Wu, J.; Peng, M.; Chen, H.H.; Camci-Unal, G.; Bayomy, A.F.; Sosnovik, D.E.; Khademhosseini, A.; et al. Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo. PLoS ONE 2012, 7, e50491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Yung Chan, S.; Common, J.E.; Amini, S.; Miserez, A.; Birgitte Lane, E.; Kang, L. Fabrication of a 3D hair follicle-like hydrogel by soft lithography. J. Biomed. Mater. Res. A 2013, 101, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Prochazka, J.; Goodwin, A.F.; Klein, O.D. Fibroblast growth factor signaling in mammalian tooth development. Odontology 2014, 102, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, M.; Tian, W. An inductive signalling network regulates mammalian tooth morphogenesis with implications for tooth regeneration. Cell Prolif. 2013, 46, 501–508. [Google Scholar] [CrossRef]
- Miyoshi, K.; Tsuji, D.; Kudoh, K.; Satomura, K.; Muto, T.; Itoh, K.; Noma, T. Generation of human induced pluripotent stem cells from oral mucosa. J. Biosci. Bioeng. 2010, 110, 345–350. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, F.; Zhang, W.; Li, Y.; Yu, M.; Nan, X.; Chen, L.; Yue, W.; Xu, X.; Pei, X. Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Eng. Part A 2012, 18, 1677–1685. [Google Scholar] [CrossRef] [Green Version]
- Kettunen, P.; Karavanova, I.; Thesleff, I. Responsiveness of developing dental tissues to fibroblast growth factors: Expression of splicing alternatives of FGFR1, -2, -3, and of FGFR4; and stimulation of cell proliferation by FGF-2, -4, -8, and -9. Dev. Genet. 1998, 22, 374–385. [Google Scholar] [CrossRef]
- Smeds, K.A.; Pfister-Serres, A.; Miki, D.; Dastgheib, K.; Inoue, M.; Hatchell, D.L.; Grinstaff, M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 2001, 54, 115–121. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Kachouie, N.; Kang, L.; Khademhosseini, A. Arraycount, an algorithm for automatic cell counting in microwell arrays. Biotechniques 2009, 47, x–xvi. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.; Hancock, M.J.; Brigham, M.D.; Khademhosseini, A. Cell confinement in patterned nanoliter droplets in a microwell array by wiping. J. Biomed. Mater. Res. A 2010, 93, 547–557. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Huang, N.W.Y.; Wong, C.X.Y.; Pan, J.; Albakr, L.; Gu, J.; Kang, L. Microstructured Hyaluronic Acid Hydrogel for Tooth Germ Bioengineering. Gels 2021, 7, 123. https://doi.org/10.3390/gels7030123
Park S, Huang NWY, Wong CXY, Pan J, Albakr L, Gu J, Kang L. Microstructured Hyaluronic Acid Hydrogel for Tooth Germ Bioengineering. Gels. 2021; 7(3):123. https://doi.org/10.3390/gels7030123
Chicago/Turabian StylePark, Sol, Naomi W. Y. Huang, Cheryl X. Y. Wong, Jing Pan, Lamyaa Albakr, Jing Gu, and Lifeng Kang. 2021. "Microstructured Hyaluronic Acid Hydrogel for Tooth Germ Bioengineering" Gels 7, no. 3: 123. https://doi.org/10.3390/gels7030123