New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali
Abstract
:1. Introduction
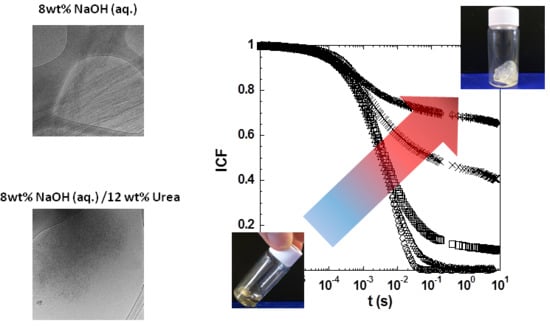
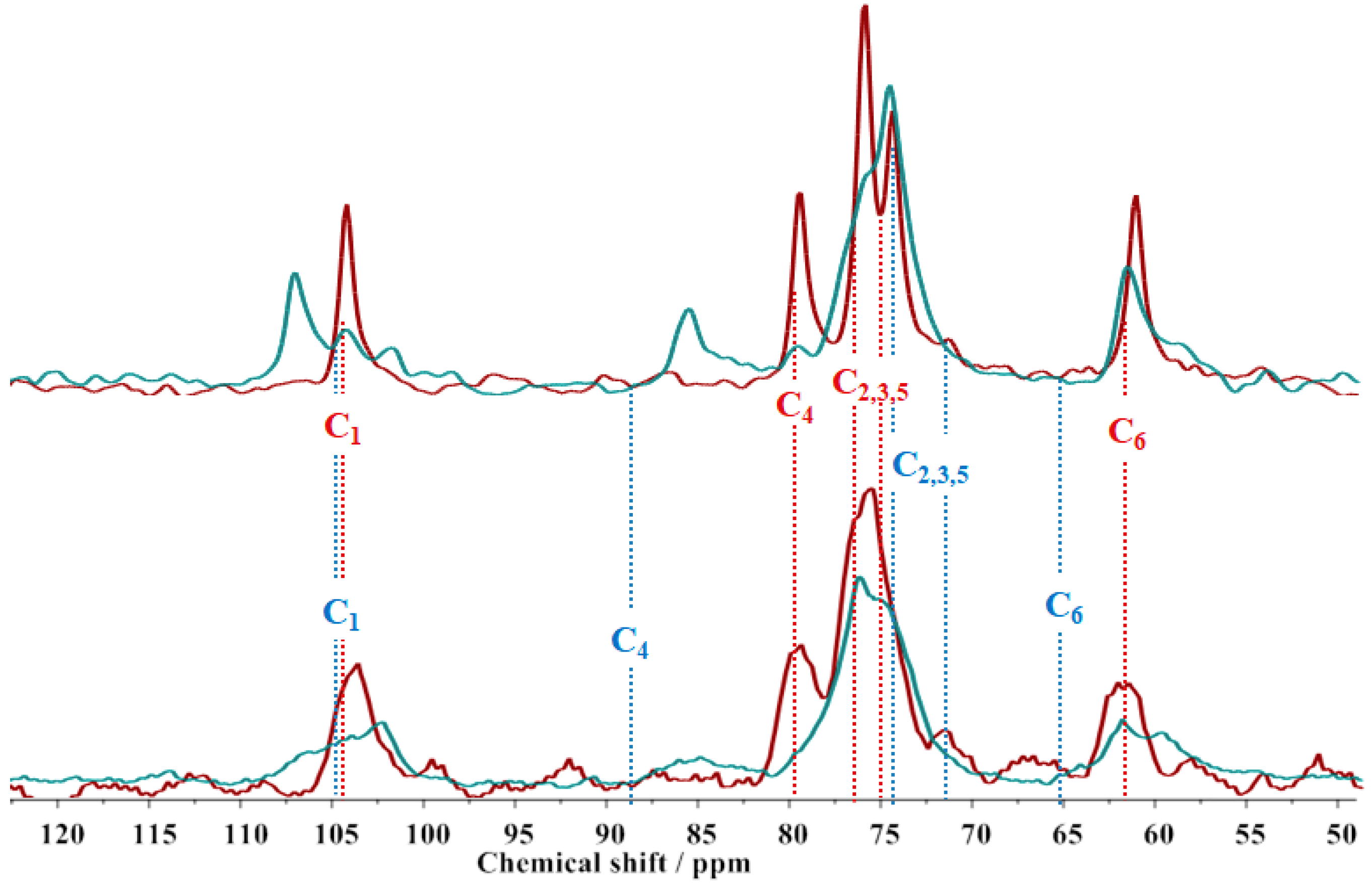
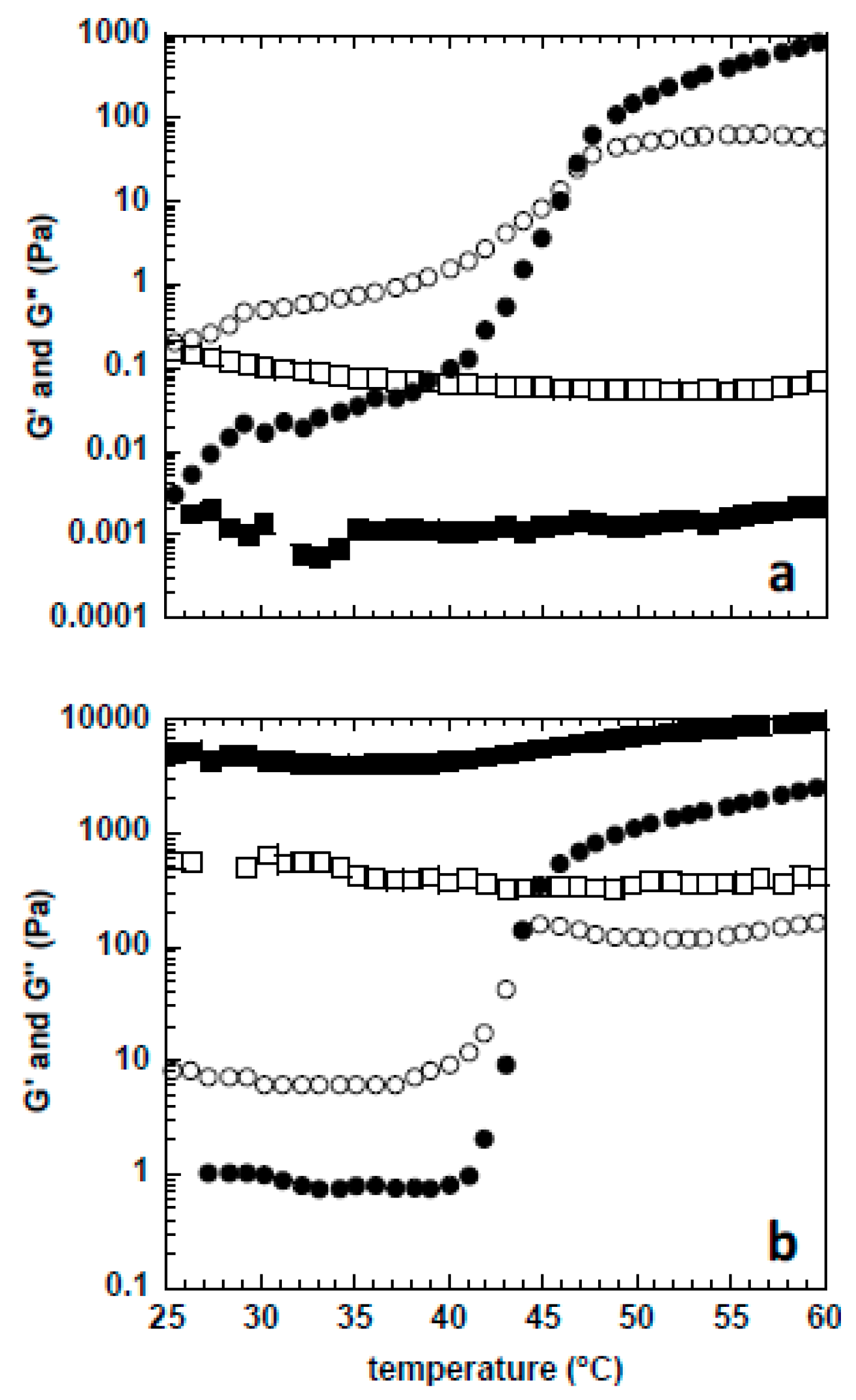
2. Results
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Sample Preparation
4.2.2. Polarization Transfer Solid-State Nuclear Magnetic Resonance
4.2.3. Cryo-Transmission Electronic Microscopy
4.2.4. Mechanical Rheometry
4.2.5. Diffusion Wave Spectroscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budtova, T.; Navard, P. Cellulose in NaOH-water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 2. Acidic solvents. Carbohydr. Polym. 2016, 151, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Medronho, B.F.; Antunes, F.E.; Romano, A.; Miguel, M.G.; Lindman, B. On the role of hydrophobic interactions in cellulose dissolution and regeneration: Colloidal aggregates and molecular solutions. Colloid Surf. A 2015, 483, 257–263. [Google Scholar] [CrossRef]
- Gubitosi, M.; Duarte, H.; Gentile, L.; Olsson, U.; Medronho, B. On cellulose dissolution and aggregation in aqueous tetrabutylammonium hydroxide. Biomacromolecules 2016, 17, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Yamane, C.; Mori, M.; Saito, M.; Okajima, K. Structures and mechanical properties of cellulose filament spun from cellulose/aqueous NaOH solution system. Polym. J. 1996, 28, 1039–1047. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents Applied in the Field of Cellulose Chemistry—A Mini Review. Polím. Ciênc. Technol. 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Gustavsson, S.; Alves, L.; Lindman, B.; Topgaard, D. Polarization transfer solid-state NMR: A new method for studying cellulose dissolution. RSC Adv. 2014, 4, 31836–31839. [Google Scholar] [CrossRef]
- Rein, D.M.; Khalfin, R.; Szekely, N.; Cohen, Y. True molecular solutions of natural cellulose in the binary ionic liquid-containing solvent mixtures. Carbohydr. Polym. 2014, 112, 125–133. [Google Scholar] [CrossRef]
- Isobe, N.; Kimura, S.; Wada, M.; Kuga, S. Mechanism of cellulose gelation from aqueous alkali-urea solution. Carbohydr. Polym. 2012, 89, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Budtova, T.; Navard, P. Rheological properties and gelation of aqueous cellulose-NaOH solutions. Biomacromolecules 2003, 4, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.H.; Zhang, L.N.; Ruan, D.; Shi, L.H.; Xu, J. Thermal gelation of cellulose in a NaOH/thiourea aqueous solution. Langmuir 2004, 20, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L. Unique gelation behavior of cellulose in NaOH/Urea aqueous solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lue, A.; Zhang, L.; Ruan, D. Inclusion complex formation of cellulose in NaOH-thiourea aqueous system at low temperature. Macromol. Chem. Phys. 2007, 208, 2359–2366. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.N.; Chang, C.Y.; Cheng, G.Z.; Chen, X.M.; Chu, B. Hydrogen-bond-induced inclusion complex in aqueous cellulose/LiOH/urea solution at low temperature. Chemphyschem 2007, 8, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Z.; Lu, A.; Cai, J.; Zhang, L.N. Stability of inclusion complex formed by cellulose in NaOH/urea aqueous solution at low temperature. Carbohydr. Polym. 2013, 92, 1315–1320. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Jin, H.J.; Zha, C.X.; Gu, L.X. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Noguchi, K.; Nishiyama, Y.; Kimura, S.; Wada, M.; Kuga, S. Role of urea in alkaline dissolution of cellulose. Cellulose 2013, 20, 97–103. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 2008, 15, 361–370. [Google Scholar] [CrossRef]
- Hagman, J.; Gentile, L.; Jessen, C.M.; Behrens, M.; Bergqvist, K.E.; Olsson, U. On the dissolution state of cellulose in cold alkali solutions. Cellulose 2017, 24, 2003–2015. [Google Scholar] [CrossRef]
- Nozaki, Y.; Tanford, C. The Solubility of Amino Acids and Related Compounds in Aqueous Urea Solutions. J. Biol. Chem. 1963, 238, 4074–4081. [Google Scholar] [PubMed]
- Zangi, R.; Zhou, R.H.; Berne, B.J. Urea’s Action on Hydrophobic Interactions. J. Am. Chem. Soc. 2009, 131, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in)solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, R.M.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef] [Green Version]
- Bergenstråhle-Wohlert, M.; Berglund, L.A.; Brady, J.W.; Larsson, P.T.; Westlund, P.O.; Wohlert, J. Concentration enrichment of urea at cellulose surfaces: Results from molecular dynamics simulations and NMR spectroscopy. Cellulose 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.E.; Romano, A.; Lindman, B. Probing Cellulose Amphiphilicity. Nordic Pulp Pap. Res. J. 2015, 30, 58–66. [Google Scholar] [CrossRef]
- Kamida, K.; Okajima, K.; Matsui, T.; Kowsaka, K. Study on the Solubility of Cellulose in Aqueous Alkali Solution by Deuteration IR and 13C NMR. Polym. J. 1984, 16, 857–866. [Google Scholar] [CrossRef]
- Porro, F.; Bédué, O.; Chanzy, H.; Heux, L. Solid-State 13C NMR Study of Na−Cellulose Complexes. Biomacromolecules 2007, 8, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Kunze, J.; Fink, H.-P. Structural Changes and Activation of Cellulose by Caustic Soda Solution with Urea. Macromol. Symp. 2005, 223, 175–188. [Google Scholar] [CrossRef]
- Pereira, A.; Duarte, H.; Nosrati, P.; Gubitosi, M.; Gentile, L.; Romano, A.; Medronho, B.; Olsson, U. Cellulose gelation in NaOH solutions is due to cellulose crystallization. Cellulose 2018, 25, 3205–3210. [Google Scholar] [CrossRef]
- Wernersson, E.; Stenqvist, B.; Lund, M. The mechanism of cellulose solubilization by urea studied by molecular simulation. Cellulose 2015, 22, 991–1001. [Google Scholar] [CrossRef]
- Romer, S.; Scheffold, F.; Schurtenberger, P. Sol-gel transition of concentrated colloidal suspensions. Phys. Rev. Lett. 2000, 85, 4980–4983. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Nutt, D.R.; Hamley, I.W.; Bucak, S.; Cenker, C.; Olsson, U. Structure of single-wall peptide nanotubes: In situ flow aligning X-ray diffraction. Chem. Commun. 2010, 46, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Bellare, J.R.; Davis, H.T.; Scriven, L.E.; Talmon, Y. Controlled environment vitrification system: An improved sample preparation technique. J. Electron. Microsc. Tech. 1988, 10, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Danev, R. Phase-plate electron microscopy: A novel imaging tool to reveal close-to-life nano-structures. Biophys. Rev. 2009, 1, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Reufer, M.; Gaudino, D.; Scheffold, F. Improved diffusing wave spectroscopy based on the automatized determination of the optical transport and absorption mean free path. Korea-Aust Rheol. J. 2017, 29, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Medronho, B.; Filipe, A.; Costa, C.; Romano, A.; Lindman, B.; Edlund, H.; Norgren, M. Microrheology of novel cellulose stabilized oil-in-water emulsions. J. Colloid Interface Sci. 2018, 531, 225–232. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, L.; Medronho, B.; Filipe, A.; E. Antunes, F.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali. Gels 2018, 4, 87. https://doi.org/10.3390/gels4040087
Alves L, Medronho B, Filipe A, E. Antunes F, Lindman B, Topgaard D, Davidovich I, Talmon Y. New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali. Gels. 2018; 4(4):87. https://doi.org/10.3390/gels4040087
Chicago/Turabian StyleAlves, Luis, Bruno Medronho, Alexandra Filipe, Filipe E. Antunes, Björn Lindman, Daniel Topgaard, Irina Davidovich, and Yeshayahu Talmon. 2018. "New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali" Gels 4, no. 4: 87. https://doi.org/10.3390/gels4040087