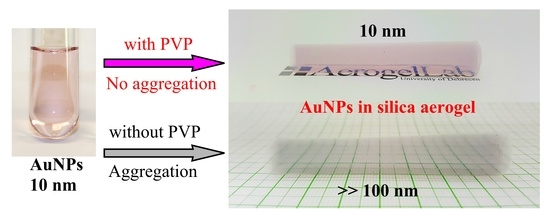
Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels
Abstract
:1. Introduction
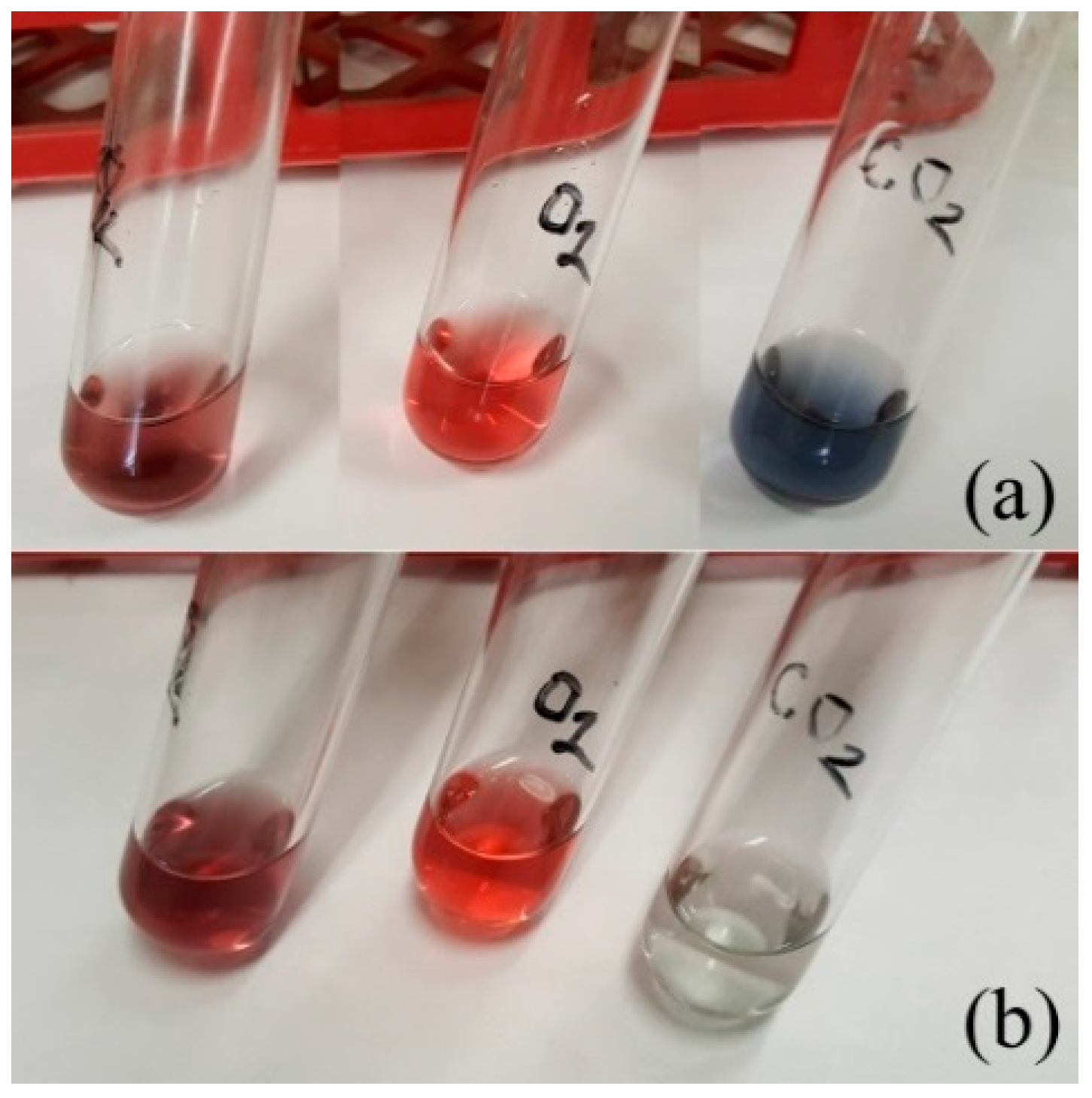
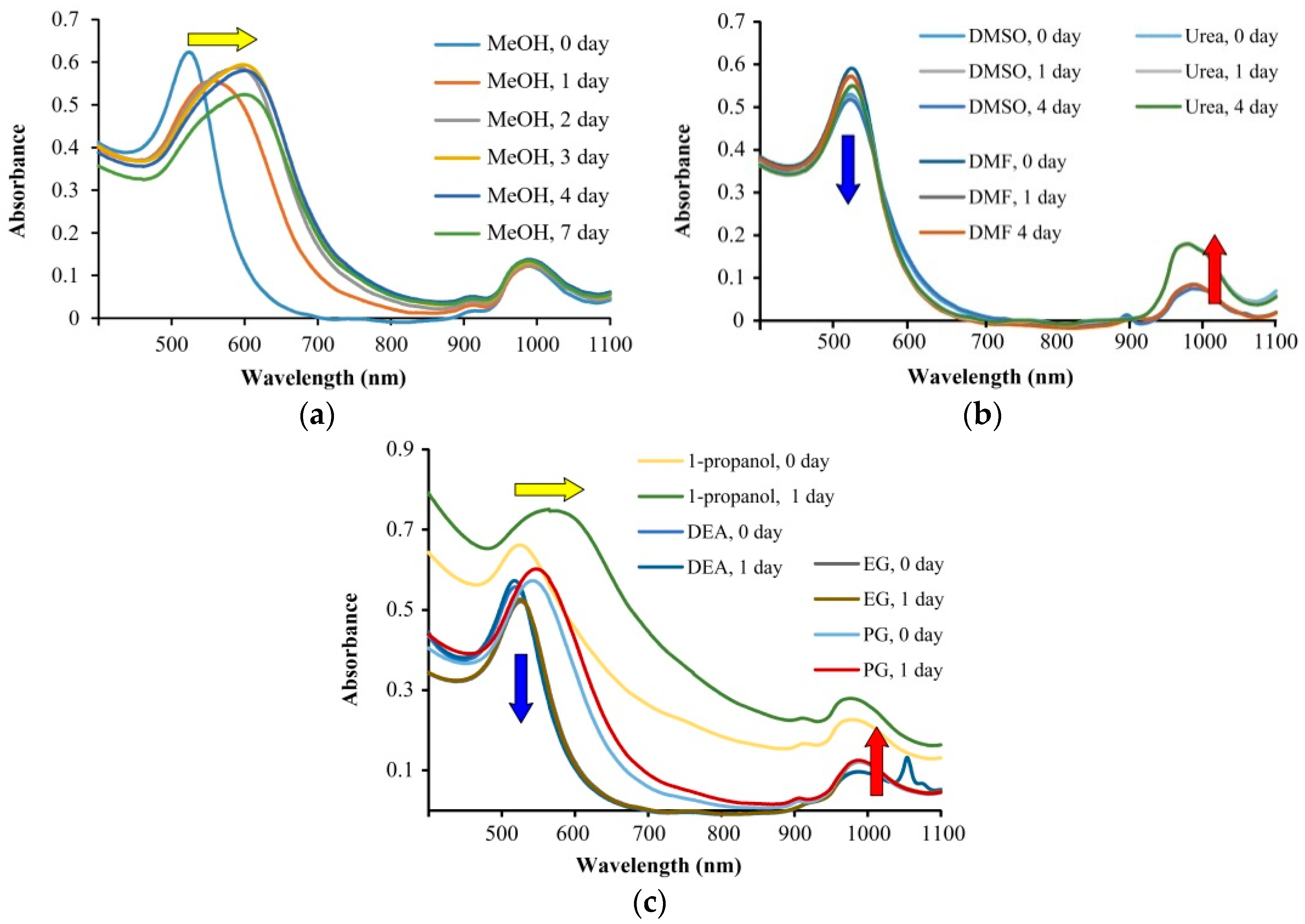
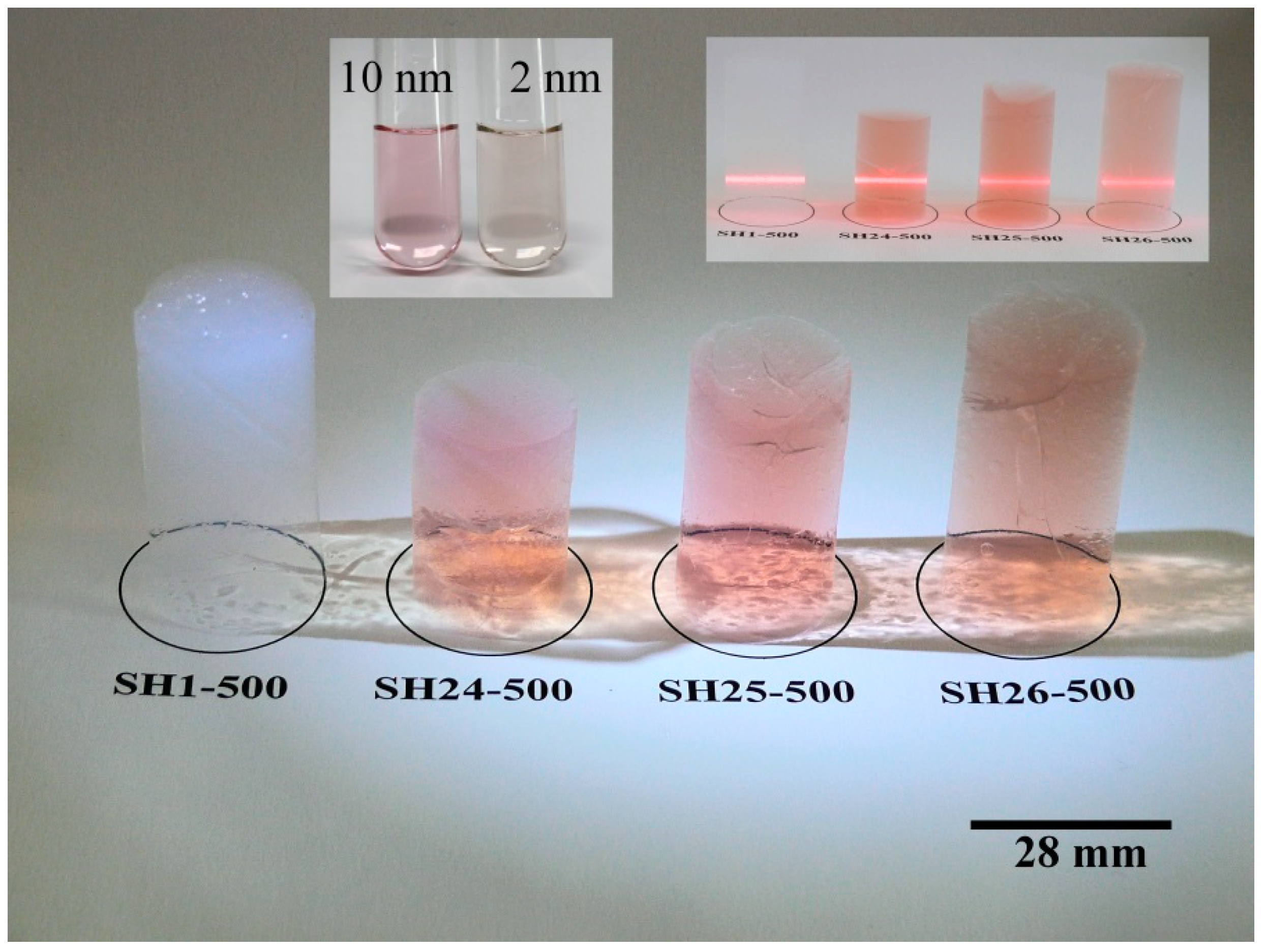
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Sol Stability Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. “Gold rush” in modern science: Fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord. Chem. Rev. 2018, 359, 1–31. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, G.P.; Bashyam, A.; Homan, K.A.; Makhija, S.; Chen, Y.-S.; Emelianov, S.Y. Silica-coated gold nanoplates as stable photoacoustic contrast agents for sentinel lymph node imaging. Nanotechnology 2013, 24, 455101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, P.P.; Yoon, S.J.; Chen, Y.-S.; Emelianov, S.; Sokolov, K.V. Development and optimization of near-IR contrast agents for immune cell tracking. Biomed. Opt. Express 2013, 4, 2609. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Huang, P.; Gao, G.; Shen, G.; Fu, S.; Cui, D.; Zhou, C.; Ren, Q. Mesoporous silica-coated gold nanorods with embedded indocyanine green for dual mode X-ray CT and NIR fluorescence imaging. Opt. Express 2011, 19, 17030. [Google Scholar] [CrossRef] [PubMed]
- Book Newell, B.; Wang, Y.; Irudayaraj, J. Multifunctional gold nanorod theragnostics probed by multi-photon imaging. Eur. J. Med. Chem. 2012, 48, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- King, S.; Massicot, J.; McDonagh, A. A Straightforward Route to Tetrachloroauric Acid from Gold Metal and Molecular Chlorine for Nanoparticle Synthesis. Metals 2015, 5, 1454–1461. [Google Scholar] [CrossRef] [Green Version]
- Philip, D. Synthesis and spectroscopic characterization of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 71, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Grewar, T.; Gericke, M. Technical Note: Synthesis and Characterization of Anisotropic Gold Nanoparticles. Adv. Nanopart. 2012, 1, 15–20. [Google Scholar] [CrossRef]
- Nagy, G.; Benkó, T.; Borkó, L.; Csay, T.; Horváth, A.; Frey, K.; Beck, A. Bimetallic Au–Ag/SiO2 catalysts: Comparison in glucose, benzyl alcohol and CO oxidation reactions. React. Kinet. Mech. Catal. 2015, 115, 45–65. [Google Scholar] [CrossRef]
- Zimbone, M.; Calcagno, L.; Baeri, P.; Messina, G.C.; Compagnini, G. Dynamic light scattering in gold colloids prepared by laser ablation in water. Appl. Surf. Sci. 2012, 258, 9246–9249. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Maniu, D.; Astilean, S. Gelatin–nanogold bioconjugates as effective plasmonic platforms for SERS detection and tagging. Colloids Surf. B Biointerfaces 2013, 103, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, Q.-Q.; Wang, J.; Wang, B.-C.; Zhang, S.; Yuan, Z. Role of thiol-containing polyethylene glycol (thiol-PEG) in the modification process of gold nanoparticles (AuNPs): Stabilizer or coagulant? J. Colloid Interface Sci. 2013, 404, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Kim, D.-H. Nanoparticle polymer composites on solid substrates for plasmonic sensing applications. Nano Today 2016, 11, 415–434. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Lim, J.; Majetich, S.A. Composite magnetic–plasmonic nanoparticles for biomedicine: Manipulation and imaging. Nano Today 2013, 8, 98–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-Gold Catalysis in Fine Chemical Synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef] [PubMed]
- Dar, B.A.; Farooqui, M. Supported nano gold as a recyclable catalyst for green, selective and efficient oxidation of alcohol using molecular oxygen. Orbital Electron. J. Chem. 2011, 3, 89–93. [Google Scholar]
- Alshammari, A.; Kalevaru, V.N. Supported Gold Nanoparticles as Promising Catalysts. In Catalytic Application of Nano-Gold Catalysts; Mishra, N.K., Ed.; InTechOpen: London, UK, 2016; pp. 57–81. ISBN 978-953-51-2640-9. [Google Scholar]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, M.; Garcia, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.D.; Rollings, M.D.; Xiao, L.; Wadsworth, W.J.; England, R.; Maier, S.A.; Birks, T.A. Plasmonic Aerogel Doped with Gold Nanoparticles; OSA: Washington, DC, USA, 2010; paper JThE21. [Google Scholar] [CrossRef]
- Anderson, M.L.; Morris, C.A.; Stroud, R.M.; Merzbacher, C.I.; Rolison, D.R. Colloidal Gold Aerogels: Preparation, Properties, and Characterization. Langmuir 1999, 15, 674–681. [Google Scholar] [CrossRef]
- Hund, J.F.; Bertino, M.F.; Zhang, G.; Sotiriou-Leventis, C.; Leventis, N. Synthesis of homogeneous alloy metal nanoparticles in silica aerogels. J. Non-Cryst. Solids 2004, 350, 9–13. [Google Scholar] [CrossRef]
- Kuthirummal, N.; Dean, A.; Yao, C.; Risen, W. Photo-formation of gold nanoparticles: Photoacoustic studies on solid monoliths of Au(III)–chitosan–silica aerogels. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Emmerling, A. Aerogels—Recent Progress in Production Techniques and Novel Applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Tao, Y.; Endo, M.; Kaneko, K. A Review of Synthesis and Nanopore Structures of Organic Polymer Aerogels and Carbon Aerogels. Recent Pat. Chem. Eng. 2010, 1, 192–200. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7477-8. [Google Scholar]
- Lázár, I.; Fábián, I. A Continuous Extraction and Pumpless Supercritical CO2 Drying System for Laboratory-Scale Aerogel Production. Gels 2016, 2, 26. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Chen, Y.; Ng, K.C.; Yan, W.; Tang, Y.; Cheng, W. Ultraflexible plasmonic nanocomposite aerogel. RSC Adv. 2011, 1, 1265. [Google Scholar] [CrossRef]
- Veres, P.; López-Periago, A.M.; Lázár, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharm. 2015, 496, 360–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bakó, J.; Fábián, I.; Lázár, I.; Jenei, A.; Hegedüs, C. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázár, I.; Szabó, H.J. Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels. Gels 2018, 4, 55. https://doi.org/10.3390/gels4020055
Lázár I, Szabó HJ. Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels. Gels. 2018; 4(2):55. https://doi.org/10.3390/gels4020055
Chicago/Turabian StyleLázár, István, and Hanna Judit Szabó. 2018. "Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels" Gels 4, no. 2: 55. https://doi.org/10.3390/gels4020055
APA StyleLázár, I., & Szabó, H. J. (2018). Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels. Gels, 4(2), 55. https://doi.org/10.3390/gels4020055