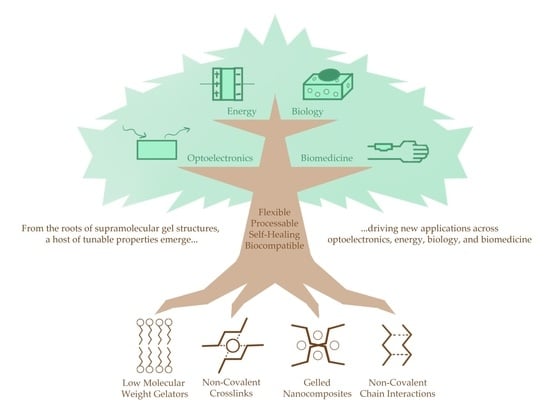
Beyond Covalent Crosslinks: Applications of Supramolecular Gels
Abstract
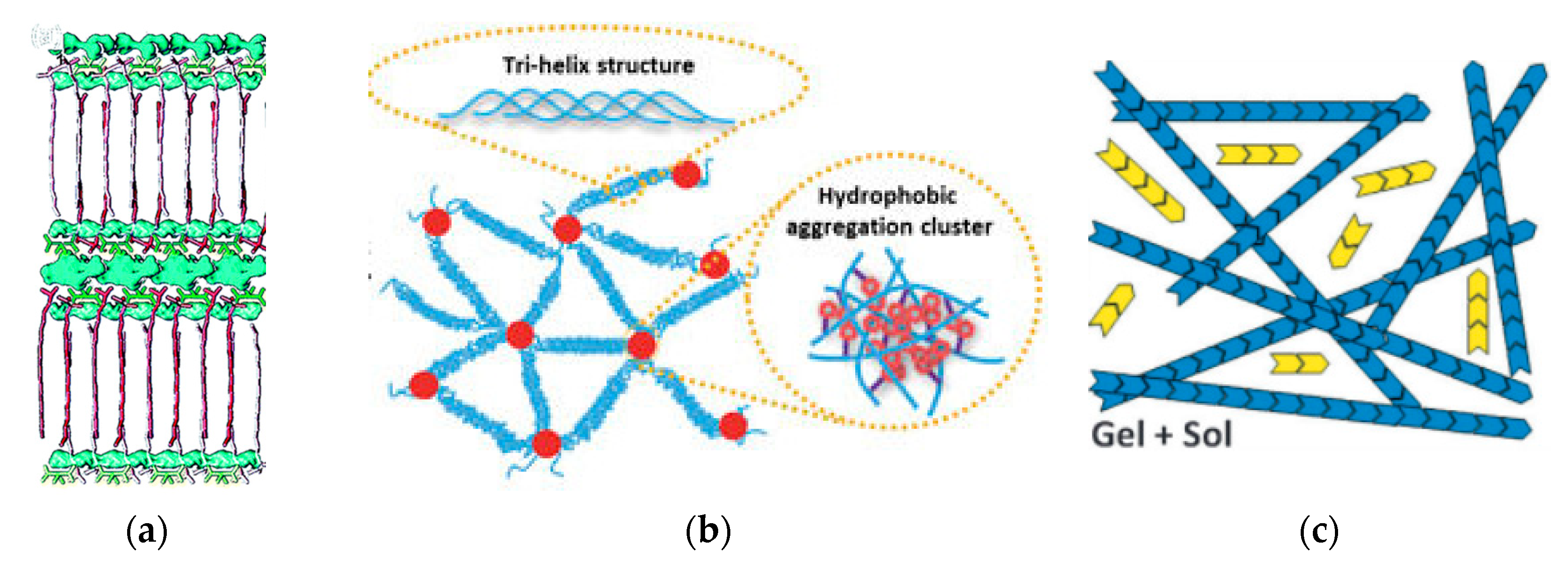
:1. Introduction
2. Supramolecular Gels for Optoelectronic Applications
2.1. Electrical Conduction
2.1.1. Flexibility
2.1.2. Processability
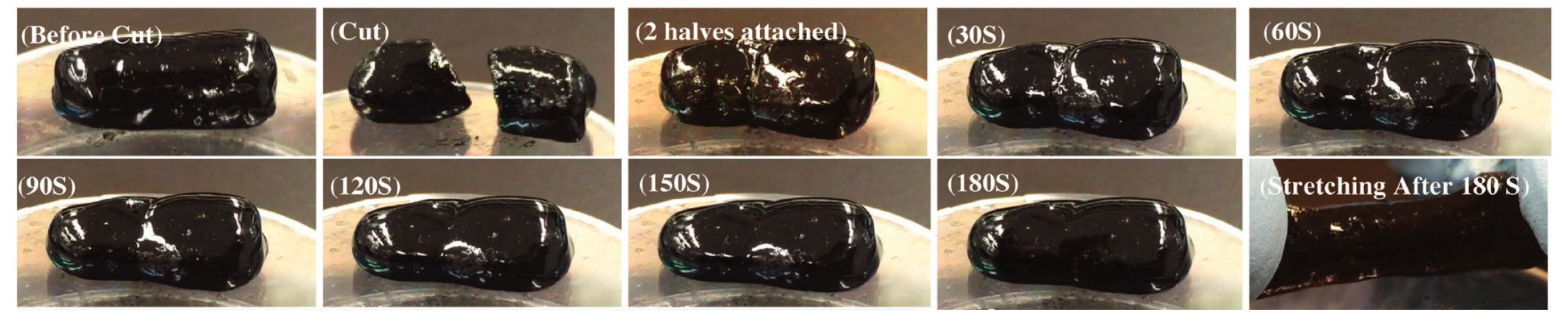
2.1.3. Self-Healing
2.2. Photonic Responses
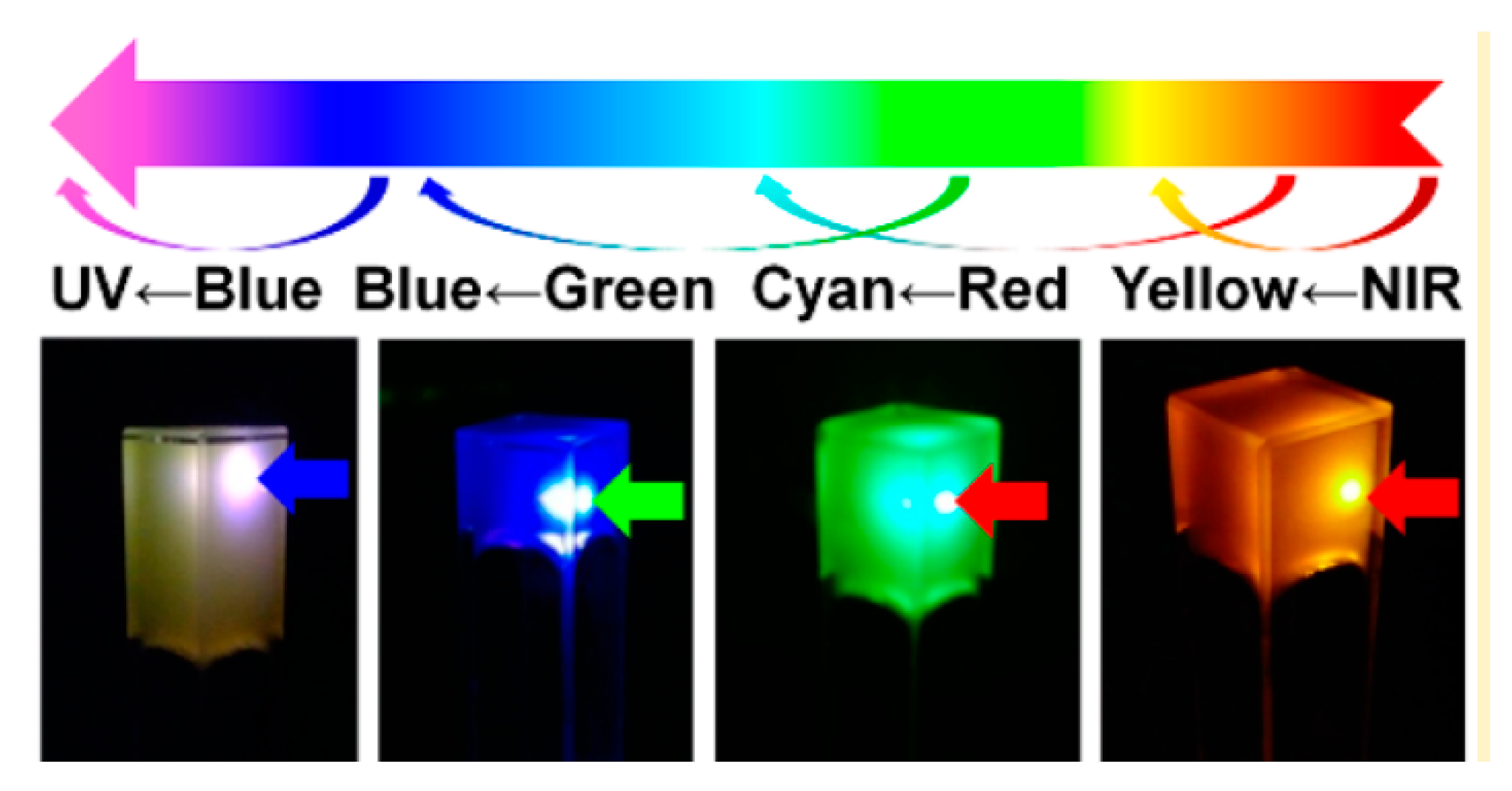
2.2.1. Luminescence
2.2.2. Scattering
2.2.3. Refraction and Absorption
2.3. Optoelectronic Devices
2.3.1. Semiconductors
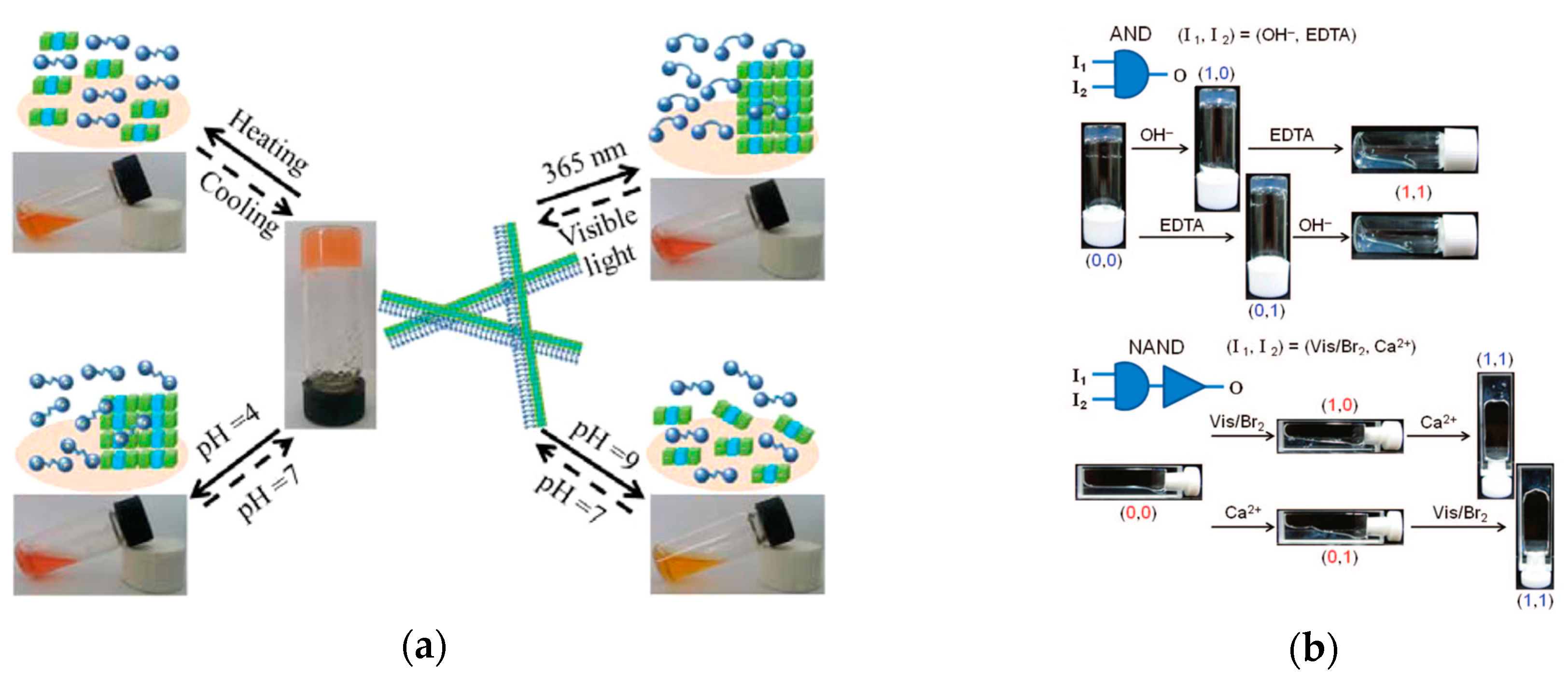
2.3.2. Logic Gates and Photo-switching
3. Supramolecular Gels for Energy Applications
3.1. Energy Generation
3.2. Energy Storage
3.2.1. Li-Ion Batteries
3.2.2. Supercapacitors
3.2.3. Fuel Cells
4. Supramolecular Gels for Biomedical Applications
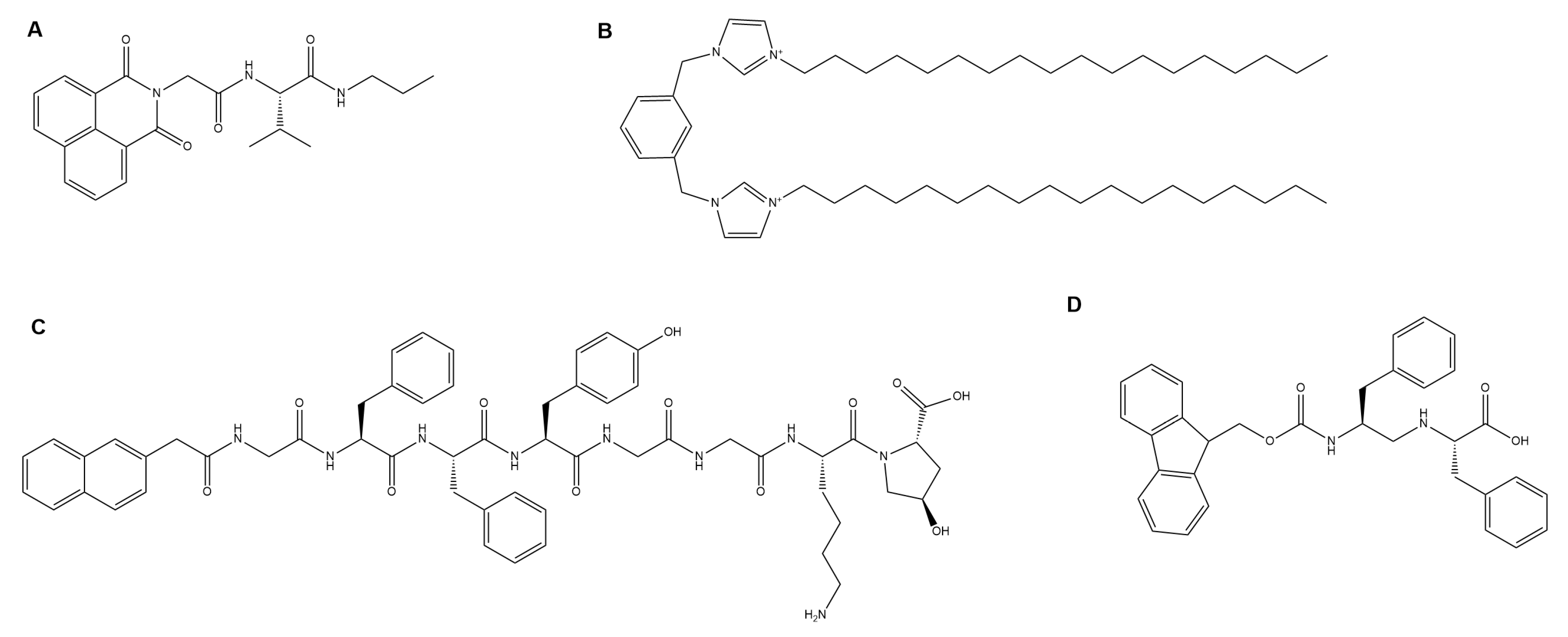
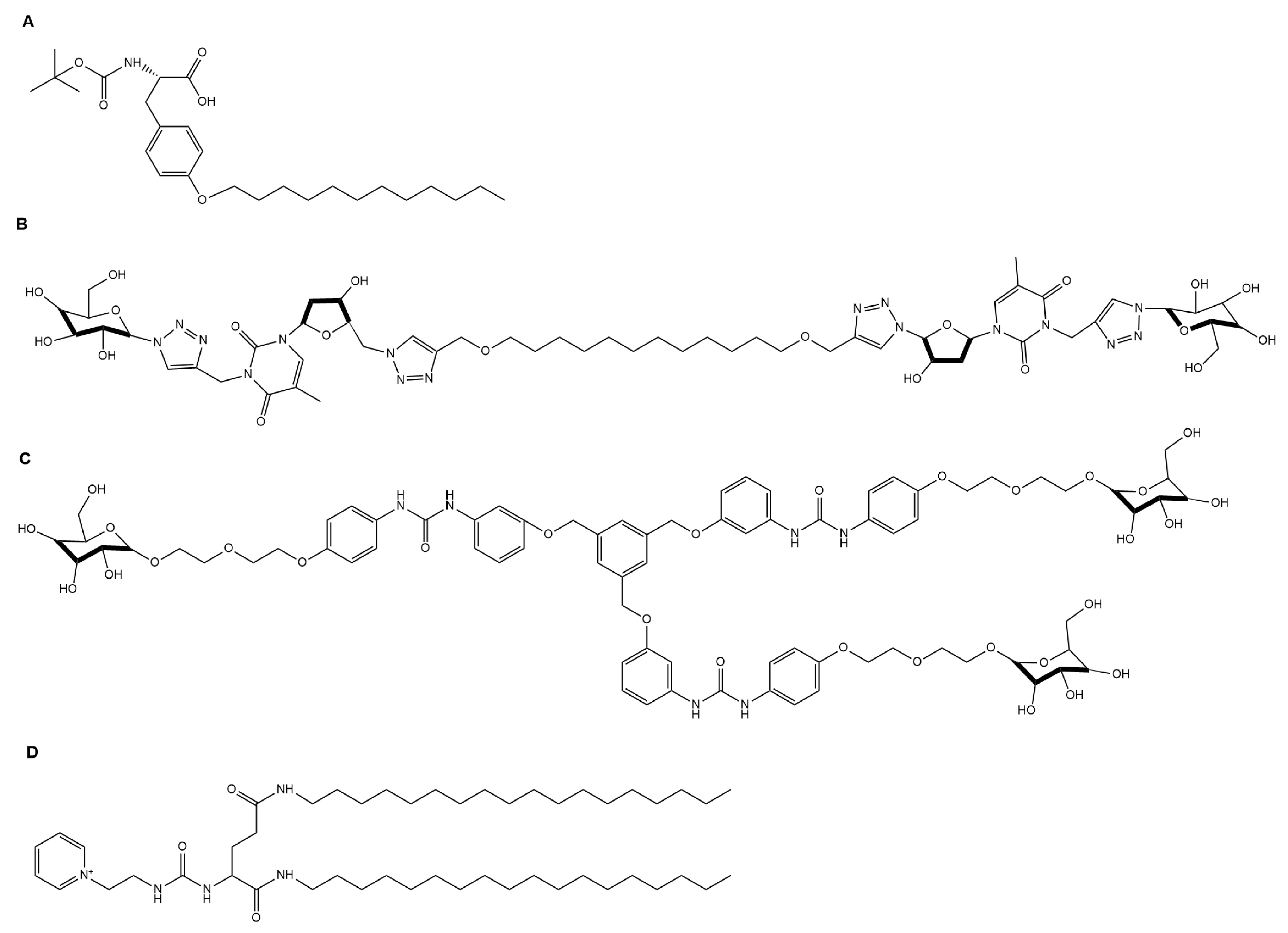
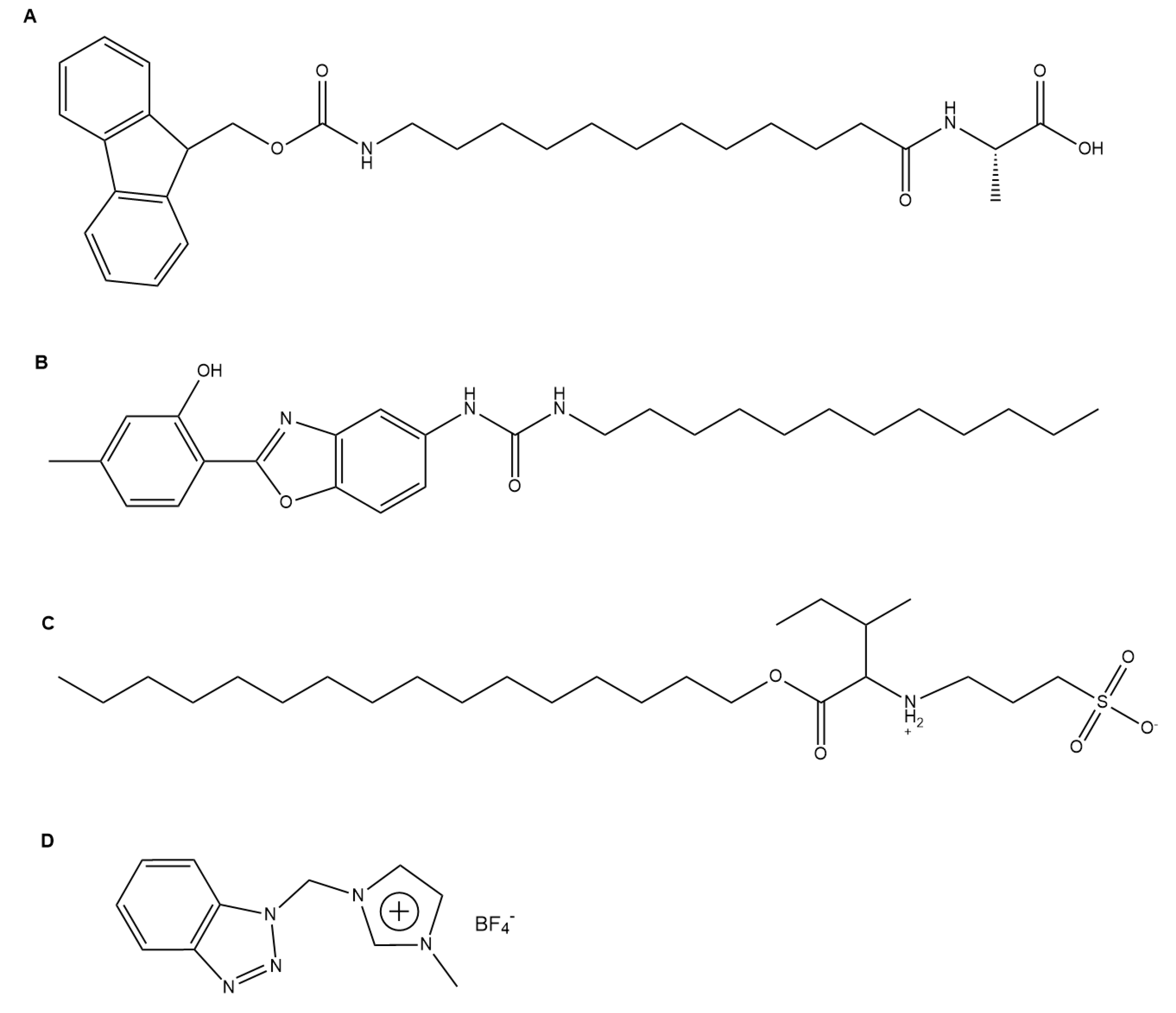
4.1. Drug Delivery
4.1.1. Oral Drug Delivery
4.1.2. Logic Gate-Based Drug Delivery
4.1.3. Topical Drug Delivery
4.1.4. Injectable Drug Delivery
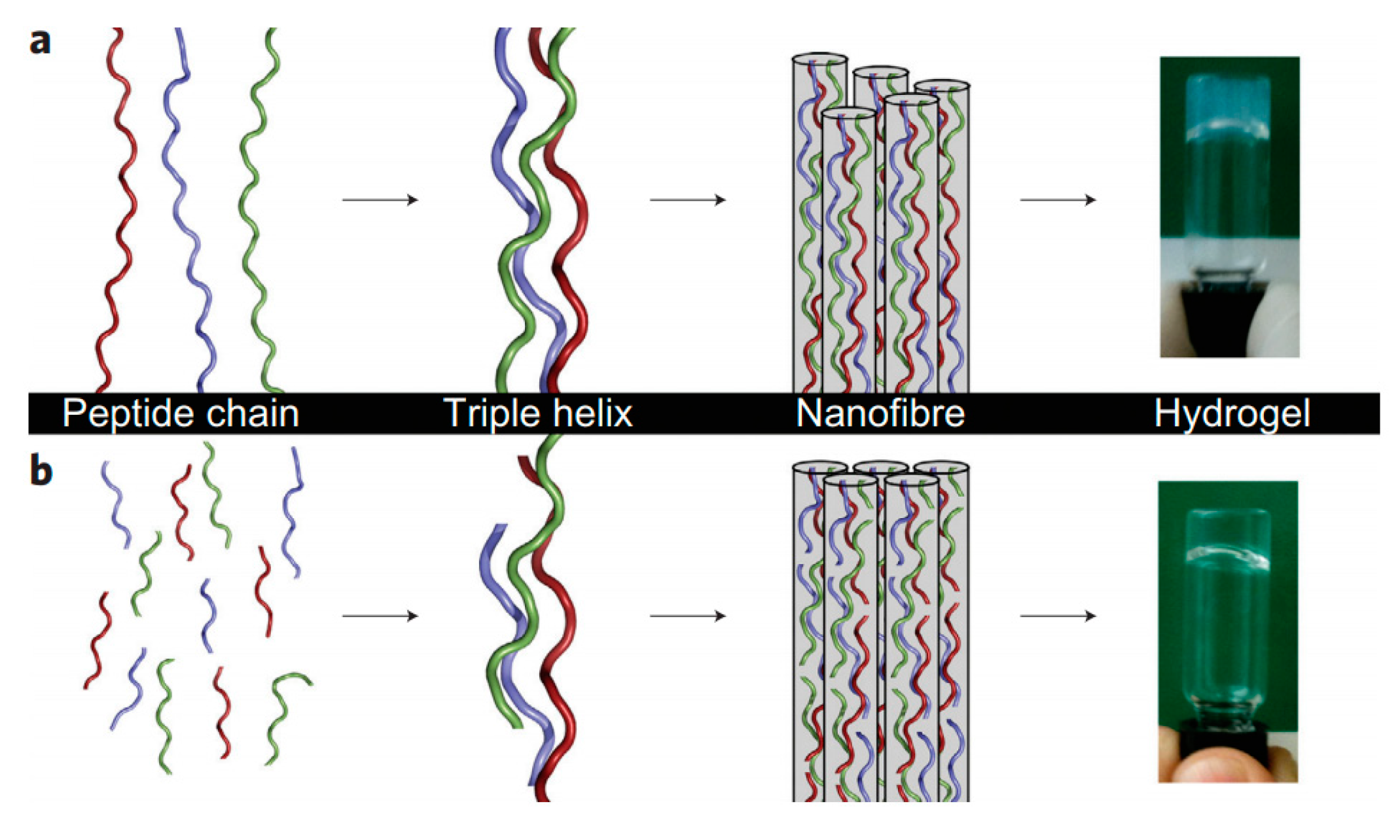
4.2. Tissue Engineering
Bone Defect Repair
4.3. Silver-Based Antimicrobial Wound Treatment
4.4. Non-Viral Vector Delivery
5. Supramolecular Gels for Biological Applications
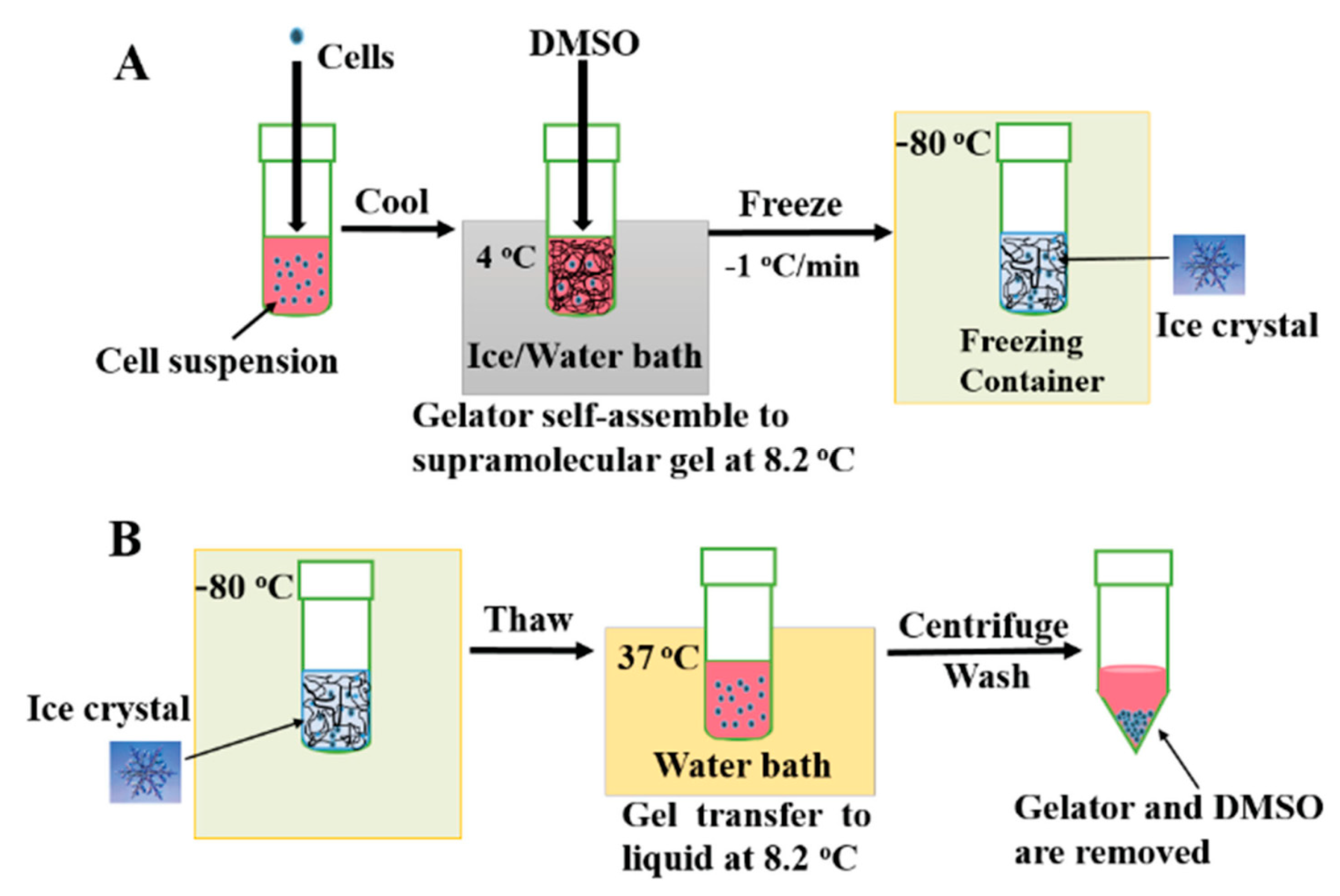
5.1. Cell Storage and Study
Stem Cell Study and Differentiation
5.2. Electrophoresis
5.3. Biological Compound Indicator
6. Special Topic Applications of Supramolecular Gels
6.1. Environmental Remediation
6.1.1. Water Treatment
6.1.2. Oil Recovery
6.1.3. Chemical Warfare Agent Sensing and Decontamination
6.2. Participation in Synthesis
6.2.1. Catalysis
6.2.2. Template for Crystal Growth
6.3. Surface Coatings
6.3.1. Tribology
6.3.2. Anticorrosion
6.4. Fat Replacement in Consumer Food Products
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Steed, J.W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Introductory lecture. Faraday Discuss. Chem. Soc. 1974, 57, 7. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels “in action”. J. Mater. Chem. 2009, 19, 6649. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821. [Google Scholar] [CrossRef] [PubMed]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089. [Google Scholar] [CrossRef] [PubMed]
- Boekhoven, J.; Brizard, A.M.; Stuart, M.C.A.; Florusse, L.; Raffy, G.; Del Guerzo, A.; van Esch, J.H. Bio-inspired supramolecular materials by orthogonal self-assembly of hydrogelators and phospholipids. Chem. Sci. 2016, 7, 6021–6031. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, K.; Zhang, K.; Yang, B.; Tian, F.; Wang, G.; Bian, L. One-pot solvent exchange preparation of non-swellable, thermoplastic, stretchable and adhesive supramolecular hydrogels based on dual synergistic physical crosslinking. NPG Asia Mater. 2018, 10, e455. [Google Scholar] [CrossRef]
- Pépin-Donat, B.; Viallat, A.; Blachot, J.F.; Lombard, C. Electromechanical polymer gels combining rubber elasticity with electronic conduction. Adv. Mater. 2006, 18, 1401–1405. [Google Scholar] [CrossRef]
- Meng, S.; Rouabhia, M.; Shi, G.; Zhang, Z. Heparin dopant increases the electrical stability, cell adhesion, and growth of conducting polypyrrole/poly(L,L-lactide) composites. J. Biomed. Mater. Res. 2008, 87, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Aida, T. Ionic liquids for soft functional materials with carbon nanotubes. Chem. Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular Ordering of Organic Molten Salts Trigged by Single-Walled Carbon Nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Lodge, T.P.; Ueki, T. Mechanically Tunable, Readily Processable Ion Gels by Self-Assembly of Block Copolymers in Ionic Liquids. Acc. Chem. Res. 2016, 49, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Aida, T. “Bucky gels” for tailoring electroactive materials and devices: The composites of carbon materials with ionic liquids. Chem. Commun. 2011, 47, 6757. [Google Scholar] [CrossRef] [PubMed]
- Kruusamäe, K.; Sugino, T.; Asaka, K. Ionic and viscoelastic mechanisms of a bucky-gel actuator. J. Appl. Phys. 2015, 118, 14502. [Google Scholar] [CrossRef]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Chun, S.J.; Lee, S.S.; Kim, B.Y.; Kim, J.H.; Chung, H.; Lee, S.Y.; Kim, W. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.B.; Jeong, K.B.; Yang, B.J.; Song, J.W.; Ku, B.C.; Lee, S.; Lee, S.K.; Park, C. Facile Supramolecular Processing of Carbon Nanotubes and Polymers for Electromechanical Sensors. Angew. Chem. 2017, 56, 16180–16185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lee, H.; Lee, E.; Kang, S.K.; Nam, J.M.; Lee, M. Responsive nematic gels from the self-assembly of aqueous nanofibres. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Pashuck, E.T.; Velichko, Y.S.; Weigand, S.J.; Cheetham, A.G.; Newcomb, C.J.; Stupp, S.I. Spontaneous and X-ray-triggered crystallization at long range in self-assembling filament networks. Science 2010, 327, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhuang, X.; Hu, J.; Lang, L.; Zhang, P.; Wang, Y.; Chen, X.; Wei, Y.; Jing, X. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules 2008, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wei, J.; Xu, B.; Liu, X.; Wang, H.; Wang, W.; Wang, Q.; Liu, W. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Andal, D.; Das, P.K. Synthesis of aLow-Molecular-Weight Fluorescent Ambidextrous Gelator: Development of Graphene-and Graphene-Oxide-Included Gel Nanocomposites. ChemPlusChem 2016, 81, 213–221. [Google Scholar] [CrossRef]
- Olive, A.G.L.; Guerzo, A.; Belin, C.; Reichwagen, J.; Hopf, H.; Desvergne, J.-P. Self-assembly of soluble anthracene, tetracene and pentacene derivatives. Res. Chem. Intermed. 2008, 34, 137–145. [Google Scholar] [CrossRef]
- Roy, S.; Maiti, D.K.; Panigrahi, S.; Basak, D.; Banerjee, A. A bolaamphiphilic amino acid appended photo-switching supramolecular gel and tuning of photo-switching behaviour. Phys. Chem. Chem. Phys. 2014, 16, 6041. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive Self-Healing Hybrid Gel Enabled by Metal-Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hashimoto, M. PVC gel soft actuator-based wearable assist wear for hip joint support during walking. Smart Mater. Struct. 2017, 26, 125003. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cao, W.T.; Ma, M.G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, Y.; Liu, S.; Wu, J. A thermo-responsive supramolecular gel and its luminescence enhancement induced by rare earth Y3+. Soft Matter 2017, 13, 8027–8030. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, J.; Tang, N.; Wu, J. A thermo-responsive supramolecular organogel: Dual luminescence properties and luminescence conversion induced by Cd2+. Dalton Trans. 2014, 43, 17236–17239. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Xiao, L.; Chen, Z.; Yan, X.; Qu, B.; Wang, S.; Gong, Q. Enhanced photoluminescence and the self-assembled fibrillar nanostructure of 5-(cholesteryloxy)methyl-8-hydroxyquinoline lithium in a gel state. New J. Chem. 2010, 34, 325–330. [Google Scholar] [CrossRef]
- Kishimura, A.; Yamashita, T.; Aida, T. Phosphorescent organogels via “metallophilic” interactions for reversible RGB-color switching. J. Am. Chem. Soc. 2005, 127, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, L.; Liu, J.; Zheng, F.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. An efficient iodide ion chemosensor and a rewritable dual-channel security display material based on an ion responsive supramolecular gel. RSC Adv. 2017, 7, 38210–38215. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Q.P.; Sun, B.; Fu, Y.P.; Zhu, X.; Wei, T.B.; Zhang, Y.M. Rewritable security display material and Cl- sensor: Based on a bimetal competitive coordination controlled supramolecular gel. Mater. Lett. 2014, 137, 444–446. [Google Scholar] [CrossRef]
- Lin, Q.; Sun, B.; Yang, Q.-P.; Fu, Y.-P.; Zhu, X.; Zhang, Y.-M.; Wei, T.-B. A novel strategy for the design of smart supramolecular gels: Controlling stimuli-response properties through competitive coordination of two different metal ions. Chem. Commun. 2014, 50, 10669–10671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Choi, H.A.; Lee, S.; Yin, J.; Kim, S.H.; Lee, C.; Yoon, J. A cholesteryl thiazolothiazole derivative for colorimetric sensing of Cu2+ and its sol-gel transition. Dyes Pigments 2015, 122, 109–115. [Google Scholar] [CrossRef]
- Fan, K.; Song, J.; Li, J.; Guan, X.; Tao, N.; Tong, C.; Shen, H.; Niu, L. Copper(II)-responsive gel-sol phase transition in supramolecular gel systems of salen-appended sorbitol. J. Mater. Chem. C 2013, 1, 7479. [Google Scholar] [CrossRef]
- Harris, R.F.; Nation, A.J.; Copeland, G.T.; Miller, S.J. A polymeric and fluorescent gel for combinatorial screening of catalysts. J. Am. Chem. Soc. 2000, 122, 11270–11271. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Lin, W.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater. 2005, 4, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, N.; He, L.; Yin, Y. Photocatalytic colour switching of redox dyes for ink-free light-printable rewritable paper. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, P.; Liu, M.; Zhou, H.; Zhang, Y.; Jiang, J.; Liu, P.; Wei, Y.; Song, Y.; Yao, X. Self-Healable Organogel Nanocomposite with Angle-Independent Structural Colors. Angew. Chem. 2017, 56, 10462–10466. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mizoshita, N.; Hanabusa, K.; Kato, T. Homeotropically oriented nematic physical gels for electrooptical materials. J. Mater. Chem. 2003, 13, 2870. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, Y.; An, B.K.; Park, S.Y. Fluorescent liquid-crystal gels with electrically switchable photoluminescence. Adv. Funct. Mater. 2006, 16, 1799–1804. [Google Scholar] [CrossRef]
- Chen, J.W.; Huang, C.C.; Chao, C.Y. Supramolecular liquid-crystal gels formed by polyfluorene-based π-conjugated polymer for switchable anisotropic scattering device. ACS Appl. Mater. Interfaces 2014, 6, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, A.; Handore, K.; Sureshan, K.M. Soft optical devices from self-healing gels formed by oil and sugar-based organogelators. Angew. Chem. 2011, 50, 8021–8024. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, A.S.; Kazantsev, R.V.; Palmer, L.C.; McClendon, M.; Koltonow, A.R.; Samuel, A.P.S.; Kiebala, D.J.; Wasielewski, M.R.; Stupp, S.I. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 2014, 6, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, R.V.; Dannenhoffer, A.J.; Weingarten, A.S.; Phelan, B.T.; Harutyunyan, B.; Aytun, T.; Narayanan, A.; Fairfield, D.J.; Boekhoven, J.; Sai, H.; et al. Crystal-Phase Transitions and Photocatalysis in Supramolecular Scaffolds. J. Am. Chem. Soc. 2017, 139, 6120–6127. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetty, R. Academic and industry research progress in germanium nanodevices. Nature 2011, 479, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vurgaftman, I.; Meyer, J.R.; Ram-Mohan, L.R. Band parameters for III-V compound semiconductors and their alloys. J. Appl. Phys. 2001, 89, 5815–5875. [Google Scholar] [CrossRef]
- Klauk, H. Organic thin-film transistors. Chem. Soc. Rev. 2010, 39, 2643. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Fréchet, J.M.J. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Sridharan, V.; Maheswari, C.U.; Nagarajan, S. Lipase catalyzed synthesis of fluorescent glycolipids: Gelation studies and graphene incorporated self-assembled sheet formation for semiconductor applications. Green Chem. 2016, 18, 3722–3731. [Google Scholar] [CrossRef]
- Horowitz, G. Organic Field-Effect Transistors. Adv. Mater. 1998, 10, 365–377. [Google Scholar] [CrossRef]
- Diring, S.; Camerel, F.; Donnio, B.; Dintzer, T.; Toffanin, S.; Capelli, R.; Muccini, M.; Ziessel, R. Luminescent ethynyl-pyrene liquid crystals and gels for optoelectronic devices. J. Am. Chem. Soc. 2009, 131, 18177–18185. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.C.; Edelsztein, V.C.; Spagnuolo, C.C.; Di Chenna, P.H.; Galian, R.E. Light-responsive hybrid material based on luminescent core-shell quantum dots and steroidal organogel. J. Mater. Chem. C 2016, 4, 7035–7042. [Google Scholar] [CrossRef]
- Jung, S.H.; Moon, J.H.; Lee, J.Y.; Jung, J.H. Thermochromic and piezochromic effects of CoII-imidazole-based supramolecular gels as logic gates. Eur. J. Inorg. Chem. 2014, 2350–2355. [Google Scholar] [CrossRef]
- Bairi, P.; Chakraborty, P.; Shit, A.; Mondal, S.; Roy, B.; Nandi, A.K. A co-assembled gel of a pyromellitic dianhydride derivative and polyaniline with optoelectronic and photovoltaic properties. Langmuir 2014, 30, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, J.; Pan, L.; Shi, Y.; Yu, G. Energy gels: A bio-inspired material platform for advanced energy applications. Nano Today 2016, 11, 738–762. [Google Scholar] [CrossRef]
- Huo, Z.; Tao, L.; Dai, S.; Zhu, J.; Zhang, C.; Chen, S.; Zhang, B. Quasi-solid-state dye sensitized solar cells using supramolecular gel electrolyte formed from two-component low molecular mass organogelators. Sci. China Mater. 2015, 58, 447–454. [Google Scholar] [CrossRef]
- Huo, Z.; Wang, L.; Tao, L.; Ding, Y.; Yi, J.; Alsaedi, A.; Hayat, T.; Dai, S. A supramolecular gel electrolyte formed from amide based co-gelator for quasi-solid-state dye-sensitized solar cell with boosted electron kinetic processes. J. Power Sources 2017, 359, 80–87. [Google Scholar] [CrossRef]
- Duan, P.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor-acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, H.; Fang, F.; Li, X.; Yang, Y. Effect of gelator structures on electrochemical properties of ionic-liquid supramolecular gel electrolytes. Electrochim. Acta 2010, 55, 2275–2279. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Monguzzi, A.; Bianchi, F.; Bianchi, A.; Mauri, M.; Simonutti, R.; Ruffo, R.; Tubino, R.; Meinardi, F. High efficiency up-converting single phase elastomers for photon managing applications. Adv. Energy Mater. 2013, 3, 680–686. [Google Scholar] [CrossRef]
- Börjesson, K.; Dzebo, D.; Albinsson, B.; Moth-Poulsen, K. Photon upconversion facilitated molecular solar energy storage. J. Mater. Chem. A 2013, 1, 8521. [Google Scholar] [CrossRef]
- Simon, Y.C.; Weder, C. Low-power photon upconversion through triplet-triplet annihilation in polymers. J. Mater. Chem. 2012, 22, 20817. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Gerber, L.C.H.; Doris, S.E.; Tsai, E.Y.; Fan, F.Y.; Qu, X.; Jain, A.; Persson, K.A.; Chiang, Y.M.; Helms, B.A. Supramolecular Perylene Bisimide-Polysulfide Gel Networks as Nanostructured Redox Mediators in Dissolved Polysulfide Lithium-Sulfur Batteries. Chem. Mater. 2015, 27, 6765–6770. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, W.; Wang, Y. Multilayer CuO@NiO Hollow Spheres: Microwave-Assisted Metal-Organic-Framework Derivation and Highly Reversible Structure-Matched Stepwise Lithium Storage. ACS Nano 2015, 9, 11462–11471. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Yu, S.; Li, M.; Wang, Z.; Nan, B.; Wu, S.; Cao, L.; Sun, Z.; Yang, M.; Wang, W.; et al. Supramolecular hydrogel directed self-assembly of C- and N-doped hollow CuO as high-performance anode materials for Li-ion batteries. Chem. Commun. 2017, 53, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.-Y.; Wang, C.; Shu, K.; Yang, Q.-S.; Ge, Y.; Wallace, G.G.; Han, B.-H. Manganese dioxide-anchored three-dimensional nitrogen-doped graphene hybrid aerogels as excellent anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 10403–10412. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, D.; Wang, W.; Zhang, L.; Xu, X. A supramolecular self-assembly hydrogel binder enables enhanced cycling of SnO2-based anode for high-performance lithium-ion batteries. J. Mater. Sci. 2017, 52, 3545–3555. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Liang, M.; Liu, X.; Li, W.; Wang, Q. A tough nanocomposite aerogel of manganese oxide and polyaniline as an electrode for a supercapacitor. Chempluschem 2016, 81, 40–43. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Wang, X.; Zhang, N.; Ma, M. Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew. Chem. 2016, 55, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells—Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Huang, Y.-J.; Cheng, C.-C.; Chang, F.-C. A new supramolecular sulfonated polyimide for use in proton exchange membranes for fuel cells. Chem. Commun. 2010, 46, 7554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.; Hashmi, S.; Karthikeyan, C.; GhavamiNejad, A.; Vatankhah-Varnoosfaderani, M.; Stadler, F.J. Graphene oxide/carbon nanotube composite hydrogels—Versatile materials for microbial fuel cell applications. Macromol. Rapid Commun. 2014, 35, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Xu, Z.; Gasteiger, H.A.; Chen, S.; Hamad-Schifferli, K.; Shao-Horn, Y. Platinum-gold nanoparticles: A highly active bifunctional electrocatalyst for rechargeable lithium-air batteries. J. Am. Chem. Soc. 2010, 132, 12170–12171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J.; Han, L.; Lin, R.; Liu, H.; Xin, H.L.; Wang, D. Supramolecular gel-assisted synthesis of double shelled Co@CoO@N-C/C nanoparticles with synergistic electrocatalytic activity for the oxygen reduction reaction. Nanoscale 2016, 8, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, C.; Shen, H.; Liu, Y.; Li, W.; Li, J. Supramolecular gel-assisted synthesis Co2P particles anchored in multielement co-doped graphene as efficient bifunctional electrocatalysts for oxygen reduction and evolution. Electrochim. Acta 2017, 231, 344–353. [Google Scholar] [CrossRef]
- Rizzo, C.; Arrigo, R.; D’Anna, F.; Di Blasi, F.; Dintcheva, N.T.; Lazzara, G.; Parisi, F.; Riela, S.; Spinelli, G.; Massaro, M. Hybrid supramolecular gels of Fmoc-F/halloysite nanotubes: Systems for sustained release of camptothecin. J. Mater. Chem. B 2017, 5, 3217–3229. [Google Scholar] [CrossRef]
- Lin, Y.; Li, L.; Li, G. A new supramolecular gel via host-guest complexation with cucurbit[8]uril and N-(4-diethylaminobenzyl)chitosan. Carbohydr. Polym. 2013, 92, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Wang, X.; Gelain, F.; Zhang, S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE 2007, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Ashwanikumar, N.; Kumar, N.A.; Saneesh Babu, P.S.; Sivakumar, K.C.; Vadakkan, M.V.; Nair, P.; Saranya, I.H.; Nair, S.A.; Vinod Kumar, G.S. Self-assembling peptide nanofibers containing phenylalanine for the controlled release of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5583–5594. [Google Scholar] [CrossRef] [PubMed]
- Felip-León, C.; Cejudo-Marín, R.; Peris, M.; Galindo, F.; Miravet, J.F. Sizing Down a Supramolecular Gel into Micro- and Nanoparticles. Langmuir 2017, 33, 10322–10328. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Calpena, A.C.; Amabilino, D.B.; Garduño-Ramírez, M.L.; Pérez-García, L. Supramolecular gels based on a gemini imidazolium amphiphile as molecular material for drug delivery. J. Mater. Chem. B 2014, 2, 5419. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Wang, J.; Wang, S.; Liao, W.; Yang, Y.; Zhang, Y.; Kong, D.; Yang, Z. Supramolecular hydrogels inspired by collagen for tissue engineering. Org. Biomol. Chem. 2010, 8, 3267. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, P.M.; Ovchinnikov, M.M.; Khizhnyak, S.D.; Roshchina, O.A.; Komarov, P.V. A supramolecular medical hydrogel based on l-cysteine and silver ions. Polym. Sci. Ser. A 2011, 53, 820–826. [Google Scholar] [CrossRef]
- Liu, G.F.; Ji, W.; Feng, C.L. Installing Logic Gates to Multiresponsive Supramolecular Hydrogel Co-assembled from Phenylalanine Amphiphile and Bis(pyridinyl) Derivative. Langmuir 2015, 31, 7122–7128. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Kurotani, K.; Onogi, S.; Urayama, K.; Hamachi, I. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel-enzyme hybrids. Nat. Chem. 2014, 6, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Matsumoto, S.; Tamaru, S.I.; Kaneko, K.; Ikeda, M.; Hamachi, I. Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release. J. Am. Chem. Soc. 2009, 131, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Limón, D.; Amirthalingam, E.; Rodrigues, M.; Halbaut, L.; Andrade, B.; Garduño-Ramírez, M.L.; Amabilino, D.B.; Pérez-García, L.; Calpena, A.C. Novel nanostructured supramolecular hydrogels for the topical delivery of anionic drugs. Eur. J. Pharm. Biopharm. 2015, 96, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Klaewklod, A.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Li, L. Characterization of supramolecular gels based on β-cyclodextrin and polyethyleneglycol and their potential use for topical drug delivery. Mater. Sci. Eng. C 2015, 50, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Deb, J.; Husain, A.; Jana, S.S.; Dastidar, P. Cetirizine derived supramolecular topical gel in action: Rational design, characterization and in vivo self-delivery application in treating skin allergy in mice. J. Mater. Chem. B 2015, 3, 6634–6644. [Google Scholar] [CrossRef]
- Marcos, X.; Pérez-Casas, S.; Llovo, J.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamer-hydroxyethyl cellulose-α-cyclodextrin supramolecular gels for sustained release of griseofulvin. Int. J. Pharm. 2016, 500, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Muthuvijayan, V.; Prasad, E. In vitro study of a glucose attached poly(aryl ether) dendron based gel as a drug carrier for a local anaesthetic. New J. Chem. 2017, 41, 7453–7462. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Xu, X.; Zhao, H.; Xue, R.; Zhou, J.; Hong, W.; Qiu, H. Fabrication of thermal sensitive folic acid based supramolecular hybrid gels for injectable drug release gels. Mater. Sci. Eng. C 2017, 75, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, K.; Wang, L.; Liu, J.; He, J.; Liu, X.; Lei, J. Injectable and thermosensitive supramolecular hydrogels by inclusion complexation between binary-drug loaded micelles and α-cyclodextrin. Mater. Sci. Eng. C 2017, 76, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.M.N.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.F.; Sandez-Macho, M.I.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable Pluronic-α-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur. J. Pharm. Biopharm. 2012, 80, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.N.; Shah, N.A.; Del Rosario Lim, M.M.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Buerkle, L.E.; von Recum, H.A.; Rowan, S.J. Toward potential supramolecular tissue engineering scaffolds based on guanosine derivatives. Chem. Sci. 2012, 3, 564–572. [Google Scholar] [CrossRef]
- Del Rosario, C.; Rodríguez-Évora, M.; Reyes, R.; Simões, S.; Concheiro, A.; Évora, C.; Alvarez-Lorenzo, C.; Delgado, A. Bone critical defect repair with poloxamine-cyclodextrin supramolecular gels. Int. J. Pharm. 2015, 495, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Huang, J.; Luo, D.; Chen, Z.; Li, X. Bone-like nanocomposites based on self-assembled protein-based matrices with Ca2+ capturing capability. J. Mater. Sci. Mater. Med. 2010, 21, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.E.R.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Meikle, S.T.; Cooper, I.R.; Lacey, J.; Perugini, V.; Santin, M. Silver-doped self-assembling di-phenylalanine hydrogels as wound dressing biomaterials. J. Mater. Sci. Mater. Med. 2013, 24, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In situ synthesised silver nanoparticle-infused l-lysine-based injectable hydrogel: Development of a biocompatible, antibacterial, soft nanocomposite. Chempluschem 2014, 79, 1733–1746. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Babicz, H.; Madry, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Cucchiarini, M. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017, 531, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.M.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Zeng, J.; Yin, Y.; Zhang, L.; Hu, W.; Zhang, C.; Chen, W. A Supramolecular Gel Approach to Minimize the Neural Cell Damage during Cryopreservation Process. Macromol. Biosci. 2016, 16, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, S.; Jinno, Y.; Haraya, N.; Oyoshi, T.; Tomitori, H.; Kashiwagi, K.; Yamanaka, M. Separation of proteins using supramolecular gel electrophoresis. Chem. Commun. 2011, 47, 10344–10346. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, C.; Zhang, L.; Liu, M. Visualized discrimination of ATP from ADP and AMP through collapse of supramolecular gels. Chem. Commun. 2014, 50, 12688–12690. [Google Scholar] [CrossRef] [PubMed]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Hu, J.; Wang, H.; Huang, J.; Yu, Y.; Zhang, Q.; Cheng, Y. Dynamic Softening or Stiffening a Supramolecular Hydrogel by Ultraviolet or Near-Infrared Light. ACS Appl. Mater. Interfaces 2017, 9, 24511–24517. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.M.; Maity, C.; Boekhoven, J.; van der Mee, L.; le Sage, V.A.A.; Groenewold, G.J.M.; van Kasteren, S.I.; Versluis, F.; van Esch, J.H.; Eelkema, R. A toolbox for controlling the properties and functionalisation of hydrazone-based supramolecular hydrogels. J. Mater. Chem. B 2016, 4, 852–858. [Google Scholar] [CrossRef]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.J.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 512. [Google Scholar] [CrossRef]
- Righetti, P.G. Electrophoresis: The march of pennies, the march of dimes. J. Chromatogr. A 2005, 1079, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M. Urea derivatives as low-molecular-weight gelators. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 33–48. [Google Scholar] [CrossRef]
- Munenobu, K.; Hase, T.; Oyoshi, T.; Yamanaka, M. Supramolecular gel electrophoresis of acidic native proteins. Anal. Chem. 2014, 86, 9924–9929. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, S.; Kobayashi, K.; Oyoshi, T.; Yamanaka, M. Supramolecular gel electrophoresis of large DNA fragments. Electrophoresis 2017, 38, 2662–2665. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, K.; Wu, M.-Y.; Liao, Y.-X.; Yu, X.-Q. Visual detection of amino acids by supramolecular gel collapse. RSC Adv. 2014, 4, 2119–2123. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, B. A simple visual assay based on small molecule hydrogels for detecting inhibitors of enzymes. Chem. Commun. 2004, 1, 2424–2425. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Nandi, N.; Mondal, B.; Dehsorkhi, A.; Hamley, I.W.; Banerjee, A. Peptide-based ambidextrous bifunctional gelator: Applications in oil spill recovery and removal of toxic organic dyes for waste water management. Interface Focus 2017, 7, 20160128. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mao, P.-P.; Fan, Y.-Q.; Liu, L.; Liu, J.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Li, C.; Jia, D.Z. A novel supramolecular polymer gel based on naphthalimide functionalized-pillar[5]arene for the fluorescence detection of Hg2+ and I− and recyclable removal of Hg2+ via cation–π interactions. Soft Matter 2017, 112, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Lv, M.; Su, C.; Zheng, L.; Li, J.; Ye, Z. An efficient supramolecular adsorbent for co-adsorption of dyes and metal ions from wastewater and its application in self-healing materials. Soft Matter 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, D.G.; Lee, M.; Lee, T.S. Synthesis of reversible fluorescent organogel containing 2-(2′-hydroxyphenyl)benzoxazole: Fluorescence enhancement upon gelation and detecting property for nerve gas simulant. Tetrahedron 2010, 66, 1667–1672. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, G.; Cai, M.; Zhou, F.; Liu, W. In situ zwitterionic supramolecular gel lubricants for significantly improved tribological properties. Tribol. Int. 2016, 95, 55–65. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Zhou, F.; Liu, W. Functional ionic gels formed by supramolecular assembly of a novel low molecular weight anticorrosive/antioxidative gelator. J. Mater. Chem. 2011, 21, 13399. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, Z.; Wang, H.; Wang, Q.; Yang, Y. Supramolecular gel composites reinforced by using halloysite nanotubes loading with in-situ formed Fe3O4 nanoparticles and used for dye adsorption. Compos. Sci. Technol. 2016, 122, 149–154. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Lei, H.; Kang, Y.; Zhao, C.; Fang, Y. Ferrocene-containing thixotropic molecular gels: Creation and a novel strategy for water purification. J. Colloid Interface Sci. 2015, 448, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Silliman, B.R.; van de Koppel, J.; McCoy, M.W.; Diller, J.; Kasozi, G.N.; Earl, K.; Adams, P.N.; Zimmerman, A.R. Degradation and resilience in Louisiana salt marshes after the BP-Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 11234–11239. [Google Scholar] [CrossRef] [PubMed]
- Kesava Raju, C.S.; Pramanik, B.; Kar, T.; Rao, P.V.C.; Choudary, N.V.; Ravishankar, R. Low molecular weight gels: Potential in remediation of crude oil spillage and recovery. RSC Adv. 2016, 6, 53415–53420. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Yan, X.; Ma, T.; Shao, L.; Liu, Y.; Guo, Z. Alkyl bicarbamates supramolecular organogelators with effective selective gelation and high oil recovery from oil/water mixtures. Chemosphere 2017, 167, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kesava Raju, C.S.; Pramanik, B.; Ravishankar, R.; Chalapathi Rao, P.V.; Sriganesh, G. Xylitol based phase selective organogelators for potential oil spillage recovery. RSC Adv. 2017, 7, 37175–37180. [Google Scholar] [CrossRef]
- Hiscock, J.R.; Piana, F.; Sambrook, M.R.; Wells, N.J.; Clark, A.J.; Vincent, J.C.; Busschaert, N.; Brown, R.C.D.; Gale, P.A. Detection of nerve agent via perturbation of supramolecular gel formation. Chem. Commun. 2013, 49, 9119. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Manna, A.; Paul, S. Rapid “naked eye” response of DCP, a nerve agent simulant: From molecules to low-cost devices for both liquid and vapour phase detection. RSC Adv. 2014, 4, 21984–21988. [Google Scholar] [CrossRef]
- Hiscock, J.R.; Sambrook, M.R.; Wells, N.J.; Gale, P.A. Detection and remediation of organophosphorus compounds by oximate containing organogels. Chem. Sci. 2015, 6, 5680–5684. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, J.R.; Bustone, G.P.; Clark, E.R. Decontamination and Remediation of the Sulfur Mustard Simulant CEES with “Off-the-Shelf” Reagents in Solution and Gel States: A Proof-of-Concept Study. ChemistryOpen 2017, 6, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Adamcik, J.; Bolisetty, S.; Handschin, S.; Mezzenga, R. Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features. Angew. Chem. 2015, 54, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, L.; Cao, H.; Wang, T.; Zhu, X.; Jiang, J.; Liu, M. Self-assembly of copper(II) ion-mediated nanotube and its supramolecular chiral catalytic behavior. Langmuir 2011, 27, 13847–13853. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Jung, S.H.; Park, S.; Park, K.-M.; Jung, J.H. A metal–organic framework gel with Cd2+ derived from only coordination bonds without intermolecular interactions and its catalytic ability. New J. Chem. 2013, 37, 2330. [Google Scholar] [CrossRef]
- Escuder, B.; Rodríguez-Llansola, F.; Miravet, J.F. Supramolecular gels as active media for organic reactions and catalysis. New J. Chem. 2010, 34, 1044. [Google Scholar] [CrossRef]
- Henisch, H.K.; García-Ruiz, J.M. Crystal growth in gels and Liesegang ring formation. II. Crystallization criteria and successive precipitation. J. Cryst. Growth 1986, 75, 203–211. [Google Scholar] [CrossRef]
- Foster, J.A.; Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.K.; Steed, J.W. Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical polymorph control in a drug-mimetic supramolecular gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Kotova, O.; Boese, M.; Gunnlaugsson, T.; Boland, J.J. Chemical nano-gardens: Growth of salt nanowires from supramolecular self-assembly gels. ACS Nano 2013, 7, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Guo, R.; Zhou, F.; Liu, W. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Cai, M.; Zhou, F.; Liu, W. Highlighting the Effect of Interfacial Interaction on Tribological Properties of Supramolecular Gel Lubricants. Adv. Mater. Interfaces 2016, 3, 1500489. [Google Scholar] [CrossRef]
- Yu, Q.; Li, D.; Cai, M.; Zhou, F.; Liu, W. Supramolecular Gel Lubricants Based on Amino Acid Derivative Gelators. Tribol. Lett. 2016, 61, 16. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Y.; Li, D.M.; Cai, M.; Zhou, F.; Liu, W. Supramolecular ionogel lubricants with imidazolium-based ionic liquids bearing the urea group as gelator. J. Colloid Interface Sci. 2017, 487, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A. Novel structuring strategies for unsaturated fats—Meeting the zero-trans, zero-saturated fat challenge: A review. Food Res. Int. 2009, 42, 747–753. [Google Scholar] [CrossRef]
- Co, E.; Marangoni, A.G. The formation of a 12-hydroxystearic acid/vegetable oil organogel under shear and thermal fields. J. Am. Oil Chem. Soc. 2013, 90, 529–544. [Google Scholar] [CrossRef]
- Alvarez-Mitre, F.M.; Mallia, V.A.; Weiss, R.G.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Self-assembly in vegetable oils of ionic gelators derived from (R)-12-hydroxystearic acid. Food Struct. 2017, 13, 56–69. [Google Scholar] [CrossRef]
- Rogers, M.A.; Spagnuolo, P.A.; Wang, T.M.; Angka, L. A potential bioactive hard-stock fat replacer comprised of a molecular gel. Food Sci. Nutr. 2017, 5, 579–587. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christoff-Tempesta, T.; Lew, A.J.; Ortony, J.H. Beyond Covalent Crosslinks: Applications of Supramolecular Gels. Gels 2018, 4, 40. https://doi.org/10.3390/gels4020040
Christoff-Tempesta T, Lew AJ, Ortony JH. Beyond Covalent Crosslinks: Applications of Supramolecular Gels. Gels. 2018; 4(2):40. https://doi.org/10.3390/gels4020040
Chicago/Turabian StyleChristoff-Tempesta, Ty, Andrew J. Lew, and Julia H. Ortony. 2018. "Beyond Covalent Crosslinks: Applications of Supramolecular Gels" Gels 4, no. 2: 40. https://doi.org/10.3390/gels4020040