Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering
Abstract
:1. Introduction
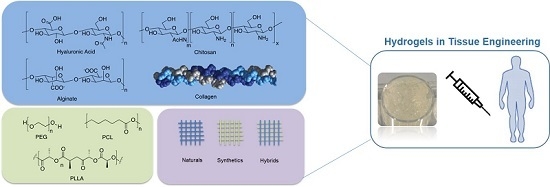
2. Classification of the Hydrogels
2.1. Natural Hydrogels
2.2. Synthetic Hydrogels
3. From Tissue Complexity to Hydrogel Design: The Simplification Game
4. Bioactivation Strategies
4.1. Hydrogel–Protein Conjugates
4.2. Hydrogel–Peptide Conjugates
4.3. Hydrogel–Glycan Conjugates
4.4. Hydrogel–Small Molecule Conjugates
5. Outlook and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, A.N.; Giuseppi-Elie, A. Bioresponsive hydrogels. Adv. Healthcare Mater. 2013, 2, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Chen, X.; Wang, W.; Loh, X.J. Engineering bioresponsive hydrogels toward healthcare applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.-J.; Hoffman, J.M.; Uto, K.; Aoyagi, T.M. Smart Biomaterials; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Carlini, A.S.; Adamiak, L.; Gianneschi, N.C. Biosynthetic polymers as functional materials. Macromolecules 2016, 49, 4379–4394. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Cipolla, L. Glycomics: New challenges and opportunities in regenerative medicine. Chem. Eur. J. 2016, 22, 13380–13388. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.C.; Marozas, I.A.; Anseth, K.S. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J. Control. Release 2015, 219, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Rafat, M.; Koh, L.B.; Islam, M.M.; Liedberg, B.O.; Griffith, M. Highly elastic epoxy cross-linked collagen hydrogels for corneal tissue engineering. Acta Ophtalmol. 2012, 90, s249. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, K.; Lin, W.; Li, D. Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J. Biomed. Mater. Res. A 2013, 101, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healtcare Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashi, R. 3D Biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Lin, C.-C. Modular cross-linking of gelatin-based thiol-norbornene hydrogels for in vitro 3D culture of hepatocellular carcinoma cells. ACS Biomater. Sci. Eng. 2015, 1, 1314–1323. [Google Scholar] [CrossRef]
- Russo, L.; Sgambato, A.; Visone, R.; Occhetta, P.; Moretti, M.; Rasponi, M.; Nicotra, F.; Cipolla, L. Gelatin hydrogels via thiol-ene chemistry. Monatsh. Chem. 2016, 147, 587–592. [Google Scholar] [CrossRef]
- Xu, K.; Cantu, D.A.; Fu, Y.; Kim, J.; Zheng, X.; Hematti, P.; Kao, W.J. Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater. 2013, 9, 8802–8814. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Lee, J.; Bae, Y.H.; Sohn, Y.S.; Jeong, B. Thermogelling aqueous solutions of alternating multiblock copolymers of poly(l-lactic acid) and poly(ethylene glycol). Biomacromolecules 2006, 7, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Ventre, M.; Netti, P.A. Controlling cell functions and fate with surfaces and hydrogels: The role of material features in cell adhesion and signal transduction. Gels 2016, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Kyburz, K.A.; Anseth, K.S. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, S.; Bi, J.; Liu, K.-K. Biomimetic three-dimensional microenvironment for controlling stem cell fate. Interface Focus 2011, 1, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, F. Engineering interpenetrating network hydrogels as biomimetic cell niche with independently tunable biochemical and mechanical properties. Biomaterials 2014, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E. Bioconjugation of hydrogels for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Sgambato, A.; Lecchi, M.; Pastori, V.; Raspanti, M.; Natalello, A.; Doglia, S.M.; Nicotra, F.; Cipolla, L. Neoglucosylated collagen matrices drive neuronal cells to differentiate. ACS Chem. Neurosci. 2014, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Russo, L.; Montesi, M.; Panseri, S.; Marcacci, M.; Caravà, E.; Raspanti, M.; Cipolla, L. Different sialoside epitopes on collagen film surfaces direct mesenchymal stem cell fate. ACS Appl. Mater. Interfaces 2016, 8, 14952–14957. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, L.; Russo, L.; Shaikh, N.; Nicotra, F. Chapter 27: Materials biofunctionalization for tissue regeneration. In Polymeric Biomaterials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; Volume 2, pp. 715–736. ISBN 9781420094725. [Google Scholar]
- Petka, W.A.; Harden, J.L.; McGrath, K.P.; Wirtz, D.; Tirrell, D.A. Reversible hydrogels from self-assembling artificial proteins. Science 1998, 281, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, D.W. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef] [PubMed]
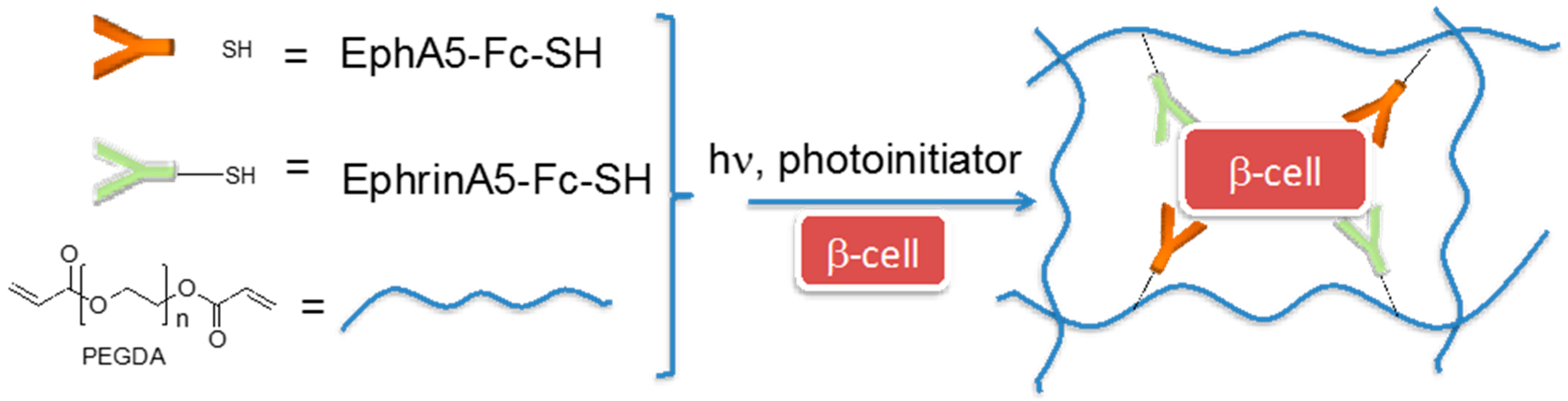
- Lin, C.C.; Anseth, K.S. Cell–cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing β-cell function. Proc. Natl. Acad. Sci. USA 2011, 108, 6380–6385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Tharp, K.M.; Ye, J.; Santiago-Ortiz, J.L.; Jackson, W.M.; Stahl, A.; Schaffer, D.V.; Yeghiazarians, Y.; Healy, K.E. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials 2015, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prokoph, S.; Chavakis, E.; Levental, K.R.; Zieris, A.; Freudenberg, U.; Dimmeler, S.; Werner, C. Sustained delivery of SDF-1α from heparin-based hydrogels to attract circulating pro-angiogenic cells. Biomaterials 2012, 33, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Rabbany, S.Y.; Pastore, J.; Yamamoto, M.; Miller, T.; Rafii, S.; Aras, R.; Penn, M. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant. 2010, 19, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Forsythe, J.S.; Parish, C.L.; Nisbet, D.R. Biofunctionalisation of polymeric scaffolds for neural tissue engineering. J. Biomater. Appl. 2012, 27, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative therapies for central nervous system diseases: A biomaterials approach. Neuropsychopharmacol. Rev. 2014, 39, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Mosiewicz, K.A.; Kolb, L.; van der Vlies, A.J.; Martino, M.M.; Lienemann, P.S.; Hubbell, J.A.; Ehrbar, M.; Lutolf, M.P. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013, 12, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, I.; Farhadi, A.; Bick, A.G.; Jabbari, E.; Khademhosseini, A. Nanoscale tissue engineering: Spatial control over cell-materials interactions. Nanotechnology 2011, 22, 212001–212017. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Madl, C.M.; Lee, S.; Duda, G.N.; Mooney, D.J. The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. J. Biomed. Mater. Res. A 2015, 103, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- DeForest, C.A.; Anseth, K.S. Cytocompatible click-based hydrogels with dynamically-tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011, 3, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, Y.J.; Lee, S.J.; Lee, S.K.; Lee, K.Y. The effect of spacer arm length of an adhesion ligand coupled to an alginate gel on the control of fibroblast phenotype. Biomaterials 2010, 31, 5545–5551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef] [PubMed]
- Miklas, J.W.; Dallabrida, S.M.; Reis, L.A.; Ismail, N.; Rupnick, M.; Radisic, M. QHREDGS enhances tube formation, metabolism and survival of endothelial cells in collagen-chitosan hydrogels. PLoS ONE 2013, 8, e72956. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Mehta, M.; Duda, G.N.; Heilshorn, S.C.; Mooney, D.J. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 2014, 15, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A 2004, 71, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Horn, D.; Schup-Magoffin, P.J.; Christman, K.L. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012, 8, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.; You, J.; Siltanen, C.; Patel, D.; Haque, A.; Anderson, L.; Revzin, A. Heparin hydrogel sandwich cultures of primary hepatocytes. Eur. Polym. J. 2015, 72, 726–735. [Google Scholar] [CrossRef]
- Li, Z.; Qu, T.; Ding, C.; Ma, C.; Sun, H.; Li, S.; Liu, X. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Lyssiotis, C.A.; Lairson, L.L.; Boitano, A.E.; Wurdak, H.; Zhu, S.; Schultz, P.G. Chemical control of stem cell fate and developmental potential. Angew. Chem. Int. Ed. 2011, 50, 200–242. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Shin, Y.M.; Joung, Y.K.; Shin, H.; Park, K.D. In situ forming hydrogels based on tyramine conjugated 4-Arm-PPO-PEO via enzymatic oxidative reaction. Biomacromolecules 2010, 11, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Prodanovic, O.; Spasojevic, D.; Prokopijevic, M.; Radotic, K.; Markovic, N.; Blazic, M.; Prodanovic, R. Tyramine modified alginates via periodate oxidation for peroxidase induced hydrogel formation and immobilization. React. Funct. Polym. 2015, 93, 77–83. [Google Scholar] [CrossRef]
- Hou, J.; Li, C.; Guan, Y.; Zhang, Y.; Zhu, X.X. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym. Chem. 2015, 6, 2204–2213. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue Adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kuang, Y.; Gao, Y.; Xu, B. Versatile small molecule motifs for self-assembly in water and formation of biofunctional supramolecular hydrogels. Langmuir 2011, 27, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hulsart-Billström, G.; Yuen, P.K.; Marsell, R.; Hilborn, J.; Larsson, S.; Ossipov, D. Bisphosphonate-linked hyaluronic acid hydrogel sequesters and enzymatically releases active bone morphogenetic protein‑2 for Induction of osteogenic differentiation. Biomacromolecules 2013, 14, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.T.H.; Aoki, T.; Sakoda, M.; Ohta, S.; Ichimura, S.; Ito, T.; Ushida, T.; Furukawa, K.S. Enhancing osteogenic differentiation of MC3T3-E1 cells by immobilizing inorganic polyphosphate onto hyaluronic acid hydrogel. Biomacromolecules 2015, 16, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.C.; Alge, D.L.; Anseth, K.S.; Bowman, C.N. A Diels-Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials 2013, 34, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mariner, P.D.; Nahreini, J.N.; Anseth, K.S. Cell-mediated delivery of glucocorticoids from thiol-ene hydrogels. J. Control. Release 2012, 162, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-healing, expansion-contraction, and shape-memory properties of a preorganized supramolecular hydrogel through host-guest interactions. Angew. Chem. Int. Ed. 2015, 54, 8984–8987. [Google Scholar] [CrossRef] [PubMed]
- Gulyuz, U.; Okay, O. Self-healing poly(acrylic acid) hydrogels with shape memory behaviour of high mechanical strength. Macromolecules 2014, 47, 6889–6899. [Google Scholar] [CrossRef]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. 2014, 45, 690–697. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels 2016, 2, 28. https://doi.org/10.3390/gels2040028
Sgambato A, Cipolla L, Russo L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels. 2016; 2(4):28. https://doi.org/10.3390/gels2040028
Chicago/Turabian StyleSgambato, Antonella, Laura Cipolla, and Laura Russo. 2016. "Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering" Gels 2, no. 4: 28. https://doi.org/10.3390/gels2040028