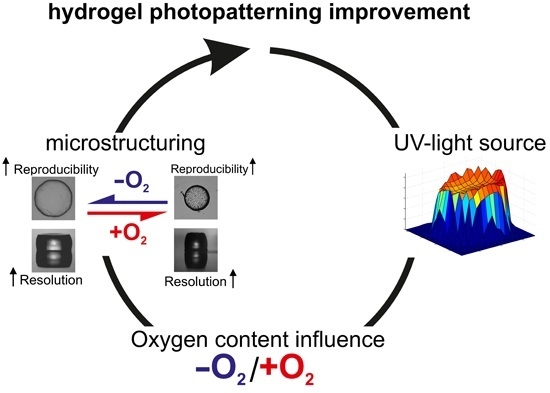
Improved PNIPAAm-Hydrogel Photopatterning by Process Optimisation with Respect to UV Light Sources and Oxygen Content
Abstract
:1. Introduction
2. Results and Discussion
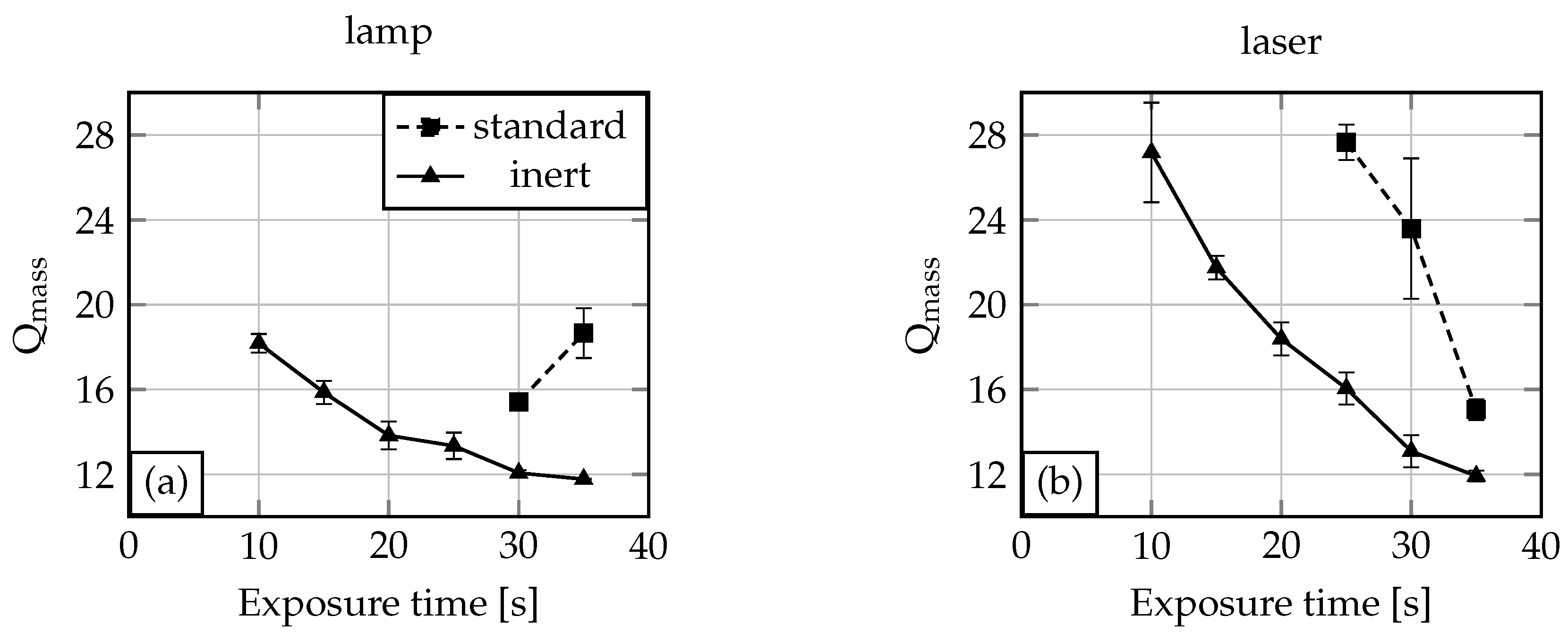
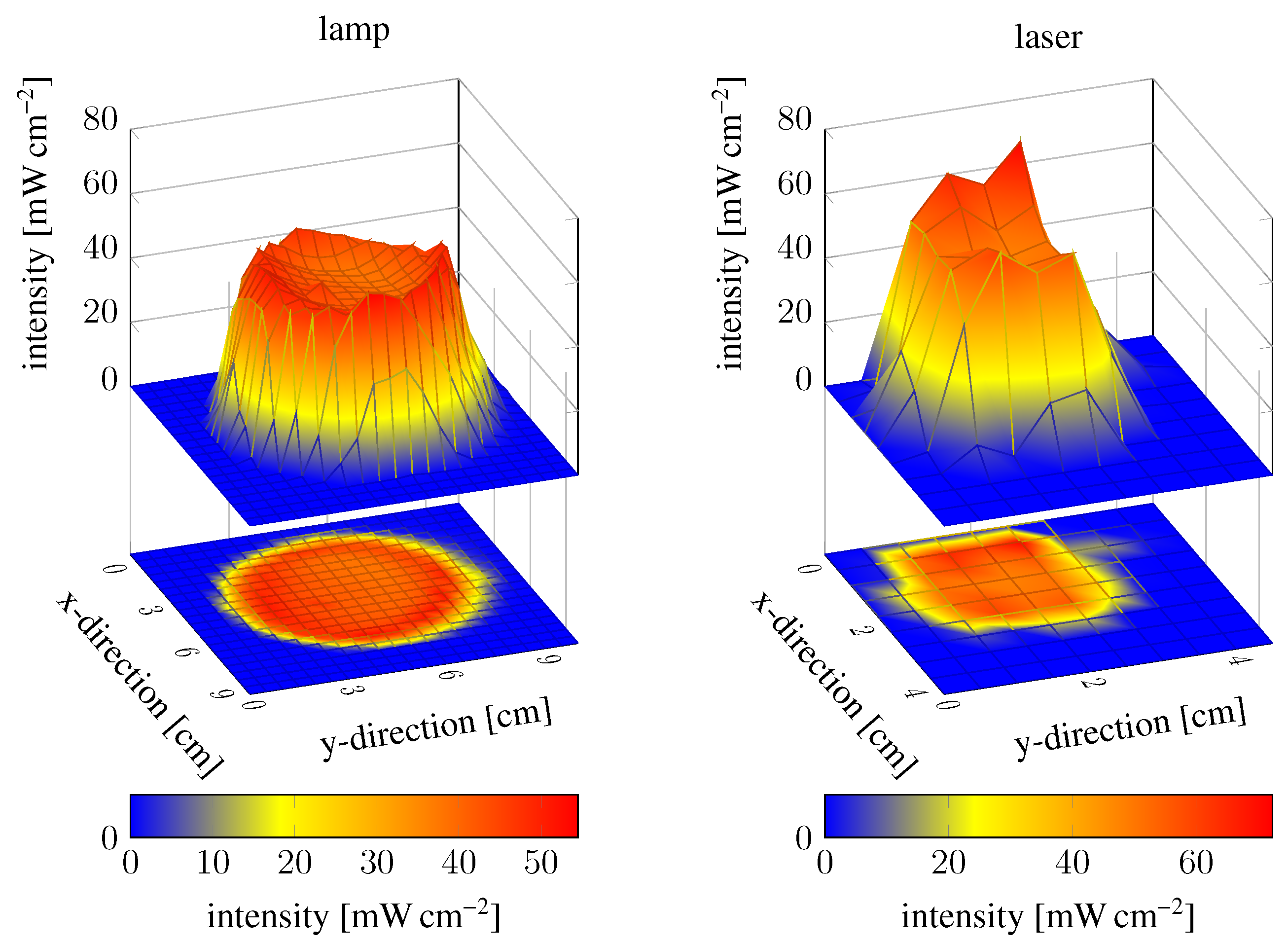
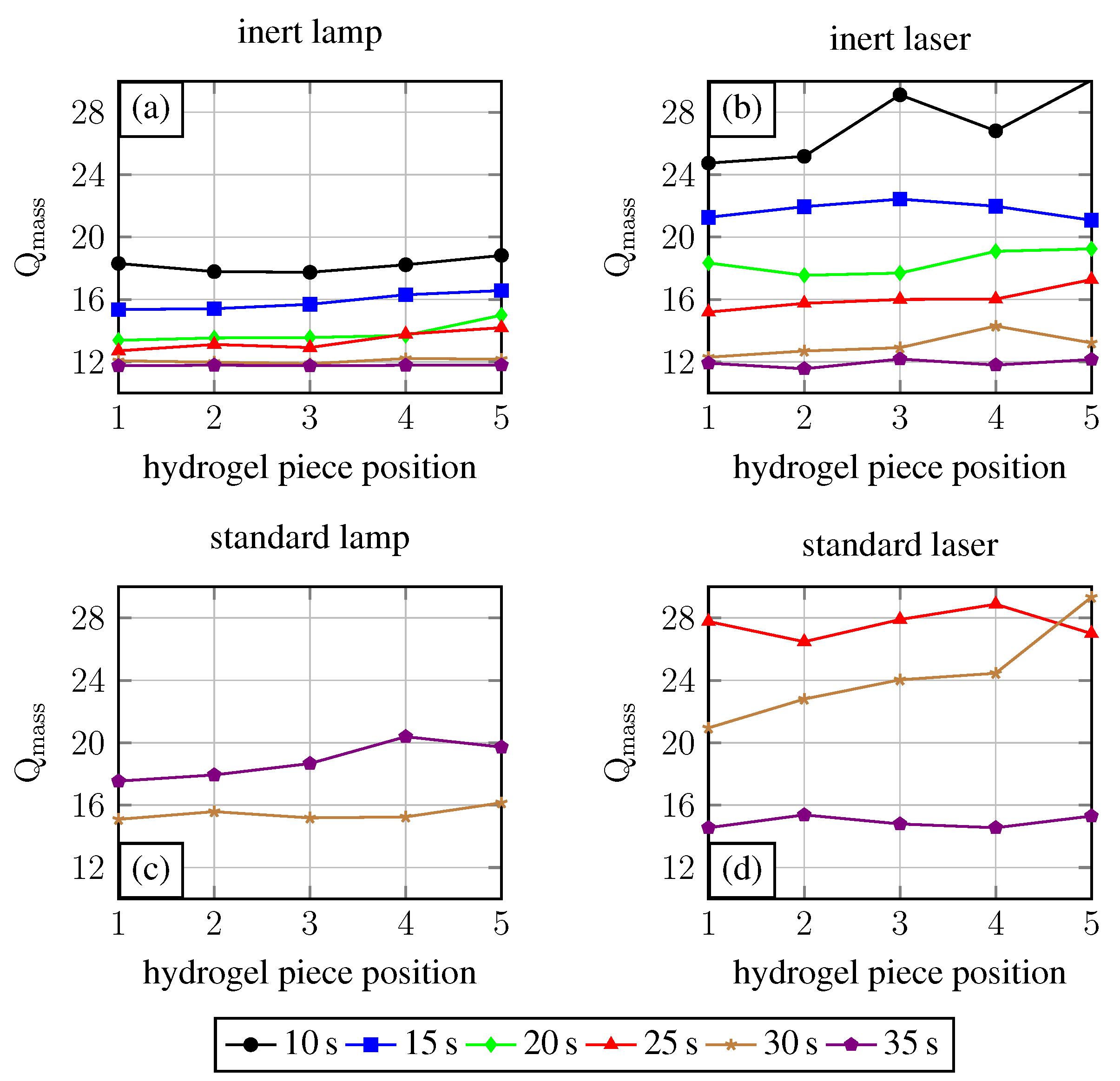
2.1. Oxygen and UV Source Influence on the Polymerisation Process
2.2. Polymerisation Process Reproducibility
2.3. Improved Hydrogel Microstructuring
3. Conclusions
4. Outlook
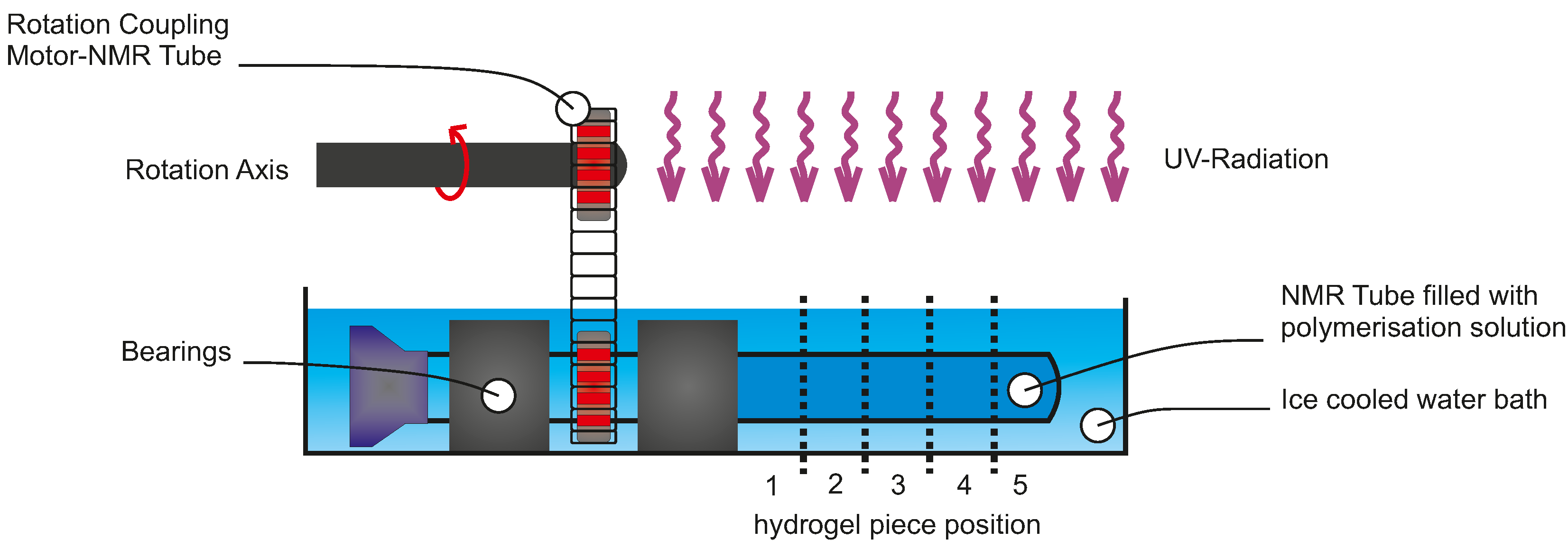
5. Experimental Section
5.1. Chemicals
5.2. Preparation of Polymerisation Solution A
5.3. Polymerisation Process Characterisation
5.4. Hydrogel Characteristics Determination—Degree of Swelling
5.5. Determination of Cooperative Diffusion Coefficient
5.6. Hydrogel Microstructuring
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. JARE 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.T.; Schultz, S.A.; Costanzo, P.J.; Martinez, A.W. Poly(N-isopropylacrylamide) hydrogels for storage and delivery of reagents to paper-based Analytical devices. Chromatography 2015, 2, 436–451. [Google Scholar] [CrossRef]
- Hynd, M.R.; Frampton, J.P.; Burnham, M.R.; Martin, D.L.; Dowell-Mesfin, N.M.; Turner, J.N.; Shain, W. Functionalized hydrogel surfaces for the patterning of multiple biomolecules. J. Biomed. Mater. Res. A. 2007, 81, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators; Urban, G., Gerlach, G., Arndt, K.-F., Eds.; Springer Series on Chemical Sensors and Biosensors 6 Berlin Heidelberg; Springer: Berlin, Germany, 2009; pp. 3–8. [Google Scholar]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Cully, M. Hydrogel drug delivery for inflammatory bowel disease. Nat. Rev. Drug Discov. 2015, 14, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 8, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Wenzel, J.; Kretschmer, K. Mechanically adjustable chemostats based on stimuli-responsive polymers. Sens. Actuators B 2007, 125, 569–573. [Google Scholar] [CrossRef]
- Richter, A.; Türke, A.; Pich, A. Controlled Double-Sensitivity of Microgels Applied to Electronically Adjustable Chemostats. Adv. Mater. 2007, 19, 1109–1112. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, P.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Richter, A.; Klenke, C.; Arndt, K.-F. Adjustable low dynamic pumps based on hydrogels. Macromol. Symp. 2004, 210, 377–384. [Google Scholar] [CrossRef]
- Richter, A.; Klatt, S.; Paschew, G.; Klenke, C. Micropumps operated by swelling and shrinking of temperature-sensitive hydrogels. Lab Chip 2009, 9, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef]
- Boyko, V.; Lu, Y.; Richter, A.; Pich, A. Preparation and characterization of acetoacetoxyethyl methacrylate- based gels. Macromol. Chem. Phys. 2003, 204, 2031–2039. [Google Scholar] [CrossRef]
- Paschew, G.; Schreiter, J.; Voigt, A.; Pini, C.; Chavez, J.P.; Allerdißen, M.; Marschner, U.; Siegmund, R.; Schüffny, R.; Jülicher, F.; et al. Autonomous chemical oscillator circuit based on bidirectional chemical-microfluidic coupling. Adv. Mater. Technol. 2015. accepted. [Google Scholar] [CrossRef]
- Greiner, R.; Allerdissen, M.; Voigt, A.; Richter, A. Fluidic microchemomechanical integrated circuits processing chemical information. Lab Chip 2012, 12, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.-F.; Krahl, F.; Richter, S.; Steiner, G. Swelling-related processes in hydrogels. In Hydrogel Sensors and Actuators; Urban, G., Gerlach, G., Arndt, K.-F., Eds.; Springer Series on Chemical Sensors and Biosensors 6 Berlin Heidelberg; Springer: Berlin, Germany, 2009; pp. 90–96. [Google Scholar]
- Shibayama, M. Spatial inhomogeneity and dynamic fluctuations of polymer gels. Macromol. Chem. Phys. 1998, 199, 1–30. [Google Scholar] [CrossRef]
- Joosten, J.G.H.; Geladé, E.T.F.; Pusey, P.N. Spatial inhomogeneity and dynamic fluctuations of polymer gels. Phys. Rev. A 1990, 42, 2161–2173. [Google Scholar] [CrossRef] [PubMed]

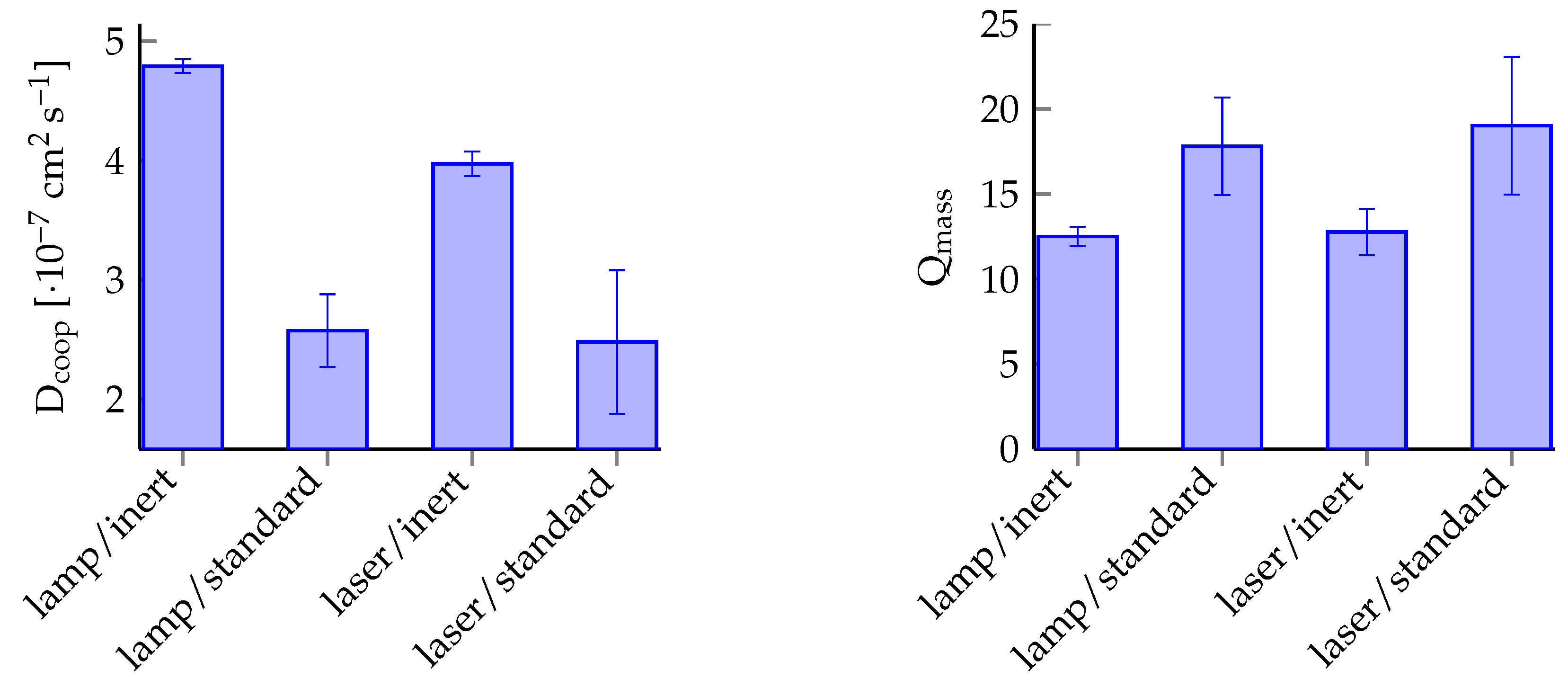

| Condition | ||||||
|---|---|---|---|---|---|---|
| Unit | (cms) | (cms) | % | (-) | (-) | % |
| lamp, inert | 4.79 × 10−7 | 5.64 × 10−9 | 1.18 | 12.50 | 0.58 | 4.61 |
| lamp, standard | 2.57 × 10−7 | 3.05 × 10−8 | 11.84 | 17.81 | 2.87 | 16.11 |
| laser, inert | 3.97 × 10−7 | 1.04 × 10−8 | 2.61 | 12.76 | 1.37 | 10.70 |
| laser, standard | 2.48 × 10−7 | 6.01 × 10−8 | 24.23 | 19.02 | 4.06 | 21.33 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haefner, S.; Rohn, M.; Frank, P.; Paschew, G.; Elstner, M.; Richter, A. Improved PNIPAAm-Hydrogel Photopatterning by Process Optimisation with Respect to UV Light Sources and Oxygen Content. Gels 2016, 2, 10. https://doi.org/10.3390/gels2010010
Haefner S, Rohn M, Frank P, Paschew G, Elstner M, Richter A. Improved PNIPAAm-Hydrogel Photopatterning by Process Optimisation with Respect to UV Light Sources and Oxygen Content. Gels. 2016; 2(1):10. https://doi.org/10.3390/gels2010010
Chicago/Turabian StyleHaefner, Sebastian, Mathias Rohn, Philipp Frank, Georgi Paschew, Martin Elstner, and Andreas Richter. 2016. "Improved PNIPAAm-Hydrogel Photopatterning by Process Optimisation with Respect to UV Light Sources and Oxygen Content" Gels 2, no. 1: 10. https://doi.org/10.3390/gels2010010
APA StyleHaefner, S., Rohn, M., Frank, P., Paschew, G., Elstner, M., & Richter, A. (2016). Improved PNIPAAm-Hydrogel Photopatterning by Process Optimisation with Respect to UV Light Sources and Oxygen Content. Gels, 2(1), 10. https://doi.org/10.3390/gels2010010