Effect of Agavins and Agave Syrup Use in the Formulation of a Synbiotic Gelatin Gummy with Microcapsules of Saccharomyces Boulardii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formulation of a Gummy with Agavins and Agave Syrup
Optimization of the Formulation of the Gummy
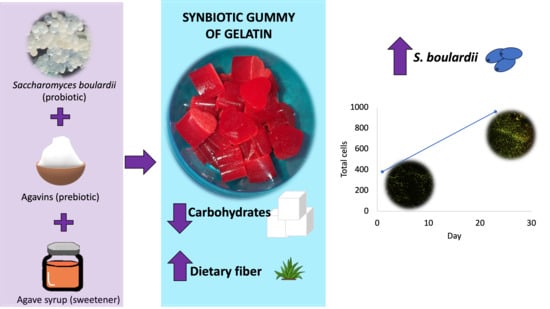
2.2. Obtaining Synbiotic Gummies
2.2.1. Physicochemical Characterization of Synbiotic Gummies
2.2.2. Viability of Microencapsulated Saccharomyces Boulardii
2.3. Morphometric Characterization of Synbiotic Gummies
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Experimental Design, Formulation and Optimization
4.3. Elaboration of Gelatin Gummies
4.3.1. Determination of Texture and Physicochemical Properties
4.4. Preparation of Synbiotic Gummies
4.4.1. Growth Conditions of Saccharomyces boulardii
4.4.2. Microencapsulation
4.4.3. Preparation of Synbiotic Gelatin Gummies
4.5. Physicochemical Properties of Synbiotic Gelatin Gummies
4.6. Determination of Viability
4.7. Digital Image Analysis
4.7.1. Viability Analysis
4.7.2. Microstructure Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INEGI (Instituto Nacional de Estadística y Geografía); ASCHOCO-CONFIMEX (Asociación Nacional de Fabricantes de Chocolates Dulces y Similares, A. C). Conociendo La Industria Del Chocolate y La Confitería. In Colección de Estudios Sectoriales y Regionales; INEGI: Aguascalientes, Mexico, 2023; pp. 1–41. [Google Scholar]
- LEGISCOMEX. Confitería en México/Inteligencia de Mercados; LEGISCOMEX: Bogota, Colombia, 2009. [Google Scholar]
- WHO (World Health Organization). Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Pérez-Cabrera, L.E.; Reyes-Bernal, K.; Godines-Hoyos, A.; Casillas-Peñuelas, R. Desarrollo y Caracterización de Golosinas Con Ingredientes de Interés Nutrimental. CienciaUAT 2012, 6, 50. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R. Golosinas Funcionales: Satisfacción Saludable Para Los Golosos; Mundo Alimentario: Mexico City, Mexico, 2010; pp. 28–31. [Google Scholar]
- Wang, Y. Prebiotics: Present and Future in Food Science and Technology. Food Res. Int. 2009, 42, 8–12. [Google Scholar] [CrossRef]
- Icaza-Chávez, M. Microbiota Intestinal En La Salud y La Enfermedad. Rev. Gastroenterol. México 2013, 78, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- López, M.G.; Salomé-Abarca, L.F. The agavins (Agave carbohydrates) story. Carbohydr. Polym. 2024, 327, 121671. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Margalli, N.A.; López, M.G. Water-Soluble Carbohydrates and Fructan Structure Patterns from Agave and Dasylirion Species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Arias, C.; Nacional, I.P.; Londoño-Moreno, A.; Avila-Reyes, S.; Arenas-Ocampo, M.; Alamilla-Beltran, L.; Jimenez-Aparicio, A.; Camacho-Diaz, B. Evaluation of the fermentation of acetylated agave fructans (agavins), with Saccharomyces boulardii as a probiotic. Rev. Mex. Ing. Quim. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Velázquez-Martínez, J.R.; González-Cervantes, R.M.; Hernández-Gallegos, M.A.; Campos-Mendiola, R.; Jiménez-Aparicio, A.R.; Ocampo, M.L.A. Prebiotic Potential of Agave angustifolia Haw Fructans with Different Degrees of Polymerization. Molecules 2014, 19, 12660–12675. [Google Scholar] [CrossRef]
- García-Vieyra, M.I.; Del Real, A.; López, M.G. Agave Fructans: Their Effect on Mineral Absorption and Bone Mineral Content. J. Med. Food 2014, 17, 1247–1255. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; López, M.G. Agavins from Agave angustifolia and Agave potatorum Affect Food Intake, Body Weight Gain and Satiety-Related Hormones (GLP-1 and ghrelin) in Mice. Food Funct. 2014, 5, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The role of agave fructans in health and food applications: A review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics and Probiotics: Are They Functional Foods? Am. J. Clin. Nutr. 2000, 71, 1682s–1687s. [Google Scholar] [CrossRef]
- Tanaka, H. Viscoelastic phase separation in soft matter and foods. Faraday Discuss. 2012, 158, 371–406. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO FOOD AND NUTRITION PAPER 85; FAO: Rome, Italy, 2006. [Google Scholar]
- Vergara, D.M.; Ferrer-Carreras, I.; Ortega-Annló, E.; González-Sánchez, M.E. Probióticos: Más Allá de La Salud Intestinal. Nutr. Hosp. 2014, 30, 63–67. [Google Scholar] [CrossRef]
- WGO (World Gastroenterology Organisation). Directrices Mundiales de La Organización Mundial de Gastroenterología: Probióticos y Prebióticos; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Neut, C.; Mahieux, S.; Dubreuil, L. Antibiotic Susceptibility of Probiotic Strains: Is It Reasonable to Combine Probiotics with Antibiotics? Médecine Mal. Infect. 2017, 47, 477–483. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.; Serna-Saldívar, S.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in Food Processing: A Review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- Zamora-Vega, R.; Montañez-Soto, J.L.; Martínez-Flores, H.E.; Flores-Magallón, R.; Muñoz-Ruiz, C.V.; Venegas-González, J.; Ortega, T.D.J.A. Effect of Incorporating Prebiotics in Coating Materials for the Microencapsulation of Sacharomyces boulardii. Int. J. Food Sci. Nutr. 2012, 63, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Falcón, M.S.; Buitrago-Arias, C.; Avila-Reyes, S.V.; Solorza-Feria, J.; Arenas-Ocampo, M.L.; Camacho-Díaz, B.H.; Jiménez-Aparicio, A.R. Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein–Agavin–Alginate Beads under Simulated Gastrointestinal Conditions. Bioengineering 2022, 9, 460. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.K.; Patra, S.; Khan, I.; Natarajan, P.; Balasubramanian, P. Cytotoxic and Pharmacokinetic Studies of Indian Seaweed Polysaccharides for Formulating Raindrop Synbiotic Candy. Int. J. Biol. Macromol. 2020, 154, 557–566. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and Characterization of the Gummy–Supplements, Enriched with Probiotics and Prebiotics. CyTA—J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- López-Palestina, C.U.; García-García, A.; Romo, S.E.; Gutiérrez-Tlahque, J. Sucrose reduction and addition of agave syrup and inulin in gummies with strawberry and blackberry pulp: Impact on physicochemical, antioxidant, and sensory characteristics. Int. Food Res. J. 2023, 30, 1562–1571. [Google Scholar] [CrossRef]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural Ingredients-Based Gummy Bear Composition Designed According to Texture Analysis and Sensory Evaluation In Vivo. Molecules 2019, 24, 1442. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Barajas-Álvarez, P.; Morales-Hernández, N.; Camacho-Ruíz, R.M.; Espinosa-Andrews, H. Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes. Molecules 2022, 27, 4902. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wu, Y.; Woshnak, L.L.; Mitmesser, S.H. Effects of Hydrocolloids, Acids and Nutrients on Gelatin Network in Gummies. Food Hydrocoll. 2021, 113, 106549. [Google Scholar] [CrossRef]
- Hartel, R.W.; Von Elbe, J.H.; Hofberger, R. Jellies, Gummies and Licorices. In Confectionery Science and Technology; Springer: Cham, Switzerland, 2017; pp. 329–359. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA—J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M. Optical, Mechanical and sensory Properties of Based-Isomaltulose Gummy Confections. Food Biosci. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W.; Zhai, X.; Fu, W.; Sun, Y.; Wang, S. Phase separation phenomena in gelatin-glucose syrup mixtures: Microstructures and gel characterization. Food Hydrocoll. 2024, 148, 109378. [Google Scholar] [CrossRef]
- Tireki, S.; Sumnu, G.; Sahin, S. Investigation of Average Crosslink Distance and Physicochemical Properties of Gummy Candy during Storage: Effect of Formulation and Storage Temperature. Phys. Fluids 2023, 35, 053115. [Google Scholar] [CrossRef]
- Badui Dergal, S. Química de Los Alimentos, 4th ed.; Quintamar Duarte, E., Ed.; Pearson Educación: Ciudad de Mexico, Mexico, 2006; ISBN 978-607-32-1508-4. [Google Scholar]
- Wang, R.; Hartel, R.W.; Zhai, X.; Fu, W.; Sun, Y.; Wang, S. Phase separation and gelation of gelatin-glucose syrup mixtures and gummy confections: Effects of moisture content, sugars, citric acid, and citrates. Food Hydrocoll. 2024, 153, 110006. [Google Scholar] [CrossRef]
- Tireki, S.; Sumnu, G.; Sahin, S. Correlation between physical and sensorial properties of gummy confections with different formulations during storage. J. Food Sci. Technol. 2021, 58, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Aranda-González, I.; Tamayo-Dzul, O.; Barbosa-Martín, E.; Segura-Campos, M.; Moguel-Ordoñez, Y.; Betancur-Ancona, D. Desarrollo de Una Golosina Tipo ‘Gomita’ Reducida En Calorías Mediante La Sustitución de Azúcares Con Stevia Rebaudiana B. Nutr. Hosp. 2015, 31, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Kjellerup, B.V.; Xu, Z. Viable but Nonculturable (VBNC) State, an Underestimated and Controversial Microbial Survival Strategy. Trends Microbiol. 2023, 31, 1013–1023. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, S.; Lu, S.; Jiang, S.; Jiang, S.; Deng, Y.; Lu, J.; Wang, H.; Zhou, Y. Ethanol yield improvement in Saccharomyces cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta mutant and molecular mechanism exploration based on the metabolic flux and transcriptomics approaches. Microb. Cell Factories 2022, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Avila-Reyes, S.; Camacho-Diaz, B.; Acosta-Garcia, M.; Jimenez-Aparicio, A.; Hernandez-Sanchez, H. Effect of salt and sugar osmotic stress on the viability and morphology of Saccharomyces boulardii. Int. J. Environ. Agric. Biotechnol. 2016, 1, 593–602. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Sun, W.; Luan, Y.; Piao, M.; Deng, Y. Characterization and Formation Mechanisms of Viable, but Putatively Non-Culturable Brewer’s Yeast Induced by Isomerized Hop Extract. LWT—Food Sci. Technol. 2021, 155, 112974. [Google Scholar] [CrossRef]
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef]
- Gutiérrez-Pulido, H.; de la Vara Salazar, R. Análisis y Diseño de Experimentos, 3rd ed.; McGrawHill: Ciudad de Mexico, Mexico, 2012. [Google Scholar]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of Antimicrobial Gummy Candies with Addition of Bovine Colostrum, Essential Oils and Probiotics. Int. J. Food Sci. Technol. 2017, 53, 1227–1235. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- SARH (Secretaría de Agricultura y Recursos Hidráulicos); Dirección General del Fomento Ganadero; Cámara de Productos Alimenticios Elaborados con Leche, S.A. de C.V. Compañía Nestlé; Leche Industrializada CONASUPO S.A.; Productos de Leche del Bajío S. A., Carnation de México S. A. de C.V; Kem Fuds, S.A. NMX-F-083-1986; Alimentos. Determinación de Humedad en productos alimenticios; SARH: Ciudad de Mexico, Mexico, 1986. [Google Scholar]
- Norma Oficial Mexicana NOM-086-SSA1-1994; Bienes y Servicios. Alimentos y Bebidas no Alcohólicas con Modificaciones en su Composición; Especificaciones Nutrimentales; Secretaria de Salud: Ciudad de Mexico, Mexico, 1994.
- Lane, J.H.; Eynon, L. Determination of reducing sugars by means of Fehling’s solution with methylene blue as internal indicator. Ed. Rodger Lond. 1934. [Google Scholar] [CrossRef]
- González-Quijano, G.K.; Dorantes-Alvarez, L.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E.; Perea-Flores, M.d.J.; de León, A.V.; Hernández-Rodríguez, C. Halotolerance and Survival Kinetics of Lactic Acid Bacteria Isolated from Jalapeño Pepper (Capsicum annuum L.) Fermentation. J. Food Sci. 2014, 79, M1545–M1553. [Google Scholar] [CrossRef] [PubMed]





| Dependent Variable | Predicted Value * | Observed Value * |
|---|---|---|
| Hardness (N) | 0.82 | 0.78 ± 0.09 |
| Cohesiveness | 1.09 | 1.08 ± 0.13 |
| Springiness (mm) | 73.62 | 75.38 ± 0.42 |
| Adhesiveness (mJ) | 0.20 | 0.14 ± 0.03 |
| Gumminess (N) | 0.89 | 0.84 ± 0.08 |
| Water activity | 0.8193 | 0.8316 ± 0.023 |
| Dependent Variable | Low Value | D | Medium Value | D | High Value | D | s | t |
|---|---|---|---|---|---|---|---|---|
| Hardness (N) | 0.21 | 0.00 | 0.84 | 1.00 | 3.16 | 0.01 | 5.00 | 5.00 |
| Cohesiveness | 0.51 | 0.00 | 0.93 | 0.80 | 1.34 | 1.00 | 1.00 | 1.00 |
| Springiness (mm) | 54.70 | 0.00 | 65.40 | 0.50 | 76.10 | 1.00 | 1.00 | 1.00 |
| Adhesiveness (mJ) | 0.04 | 1.00 | 0.41 | 0.50 | 0.78 | 0.00 | 1.00 | 1.00 |
| Gumminess (N) | 0.20 | 0.00 | 0.84 | 1.00 | 3.20 | 0.01 | 5.00 | 5.00 |
| Water activity | 0.7524 | 1.00 | 0.8039 | 0.50 | 0.85 | 0.00 | 0.10 | 0.10 |
| Property | SIG | S4A4 | CG |
|---|---|---|---|
| Moisture content * (%) | 32.45 ± 0. 37 a | 34.45 ± 0.11 b | 20–25 ** c |
| Aw * | 0.7465 ± 0.01 a | 0.8518 ± 0.004 b | 0.6572 ± 0.005 c |
| Total sugars * | 28.03 ± 3.35 a | 32.78 ± 2.74 a | 51.5 ** b |
| Reducing sugars * (%) | 11.76 ± 0.29 a | 12.13 ± 0.56 a | 51.1 ** b |
| No reducing sugars * (%) | 16.26 ± 3.26 a | 20.65 ± 3.15 a | 0.4 ** b |
| Hardness (N) | 1.73 ± 0.23 a | 0.79 ± 0.07 a | 11.64 ± 1.12 b |
| Cohesiveness | 1.02 ± 0.01 a | 1.07 ± 0.02 b | 0.95 ± 0.02 c |
| Springiness (mm) | 71.43 ± 3.68 a | 75.57 ± 0.5 a | 71.95 ± 4.24 a |
| Gumminess (N) | 1.32 ± 0.66 a | 0.85 ± 0.1 a | 11.08 ± 0.81 b |
| Treatment | Agave Syrup (mL) | Agavins (g) | Treatment Key |
|---|---|---|---|
| 1 | 30 | 30 | S3A3 |
| 2 | 30 | 50 | S3A5 |
| 3 | 50 | 30 | S5A3 |
| 4 | 50 | 50 | S5A5 |
| 5 | 40 | 40 | S4A4 * |
| 6 | 26 | 40 | S26A4 |
| 7 | 54 | 40 | S54A4 |
| 8 | 40 | 26 | S4A26 |
| 9 | 40 | 54 | S4A54 |
| 10 | 40 | 40 | S4A4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigil-Cuate, L.K.; Avila-Reyes, S.V.; Camacho-Díaz, B.H.; Hernández-Sánchez, H.; Osorio-Díaz, P.; Jiménez-Aparicio, A.R.; Robert, P.; Arenas-Ocampo, M.L. Effect of Agavins and Agave Syrup Use in the Formulation of a Synbiotic Gelatin Gummy with Microcapsules of Saccharomyces Boulardii. Gels 2024, 10, 299. https://doi.org/10.3390/gels10050299
Vigil-Cuate LK, Avila-Reyes SV, Camacho-Díaz BH, Hernández-Sánchez H, Osorio-Díaz P, Jiménez-Aparicio AR, Robert P, Arenas-Ocampo ML. Effect of Agavins and Agave Syrup Use in the Formulation of a Synbiotic Gelatin Gummy with Microcapsules of Saccharomyces Boulardii. Gels. 2024; 10(5):299. https://doi.org/10.3390/gels10050299
Chicago/Turabian StyleVigil-Cuate, Liliana K., Sandra V. Avila-Reyes, Brenda H. Camacho-Díaz, Humberto Hernández-Sánchez, Perla Osorio-Díaz, Antonio R. Jiménez-Aparicio, Paz Robert, and Martha L. Arenas-Ocampo. 2024. "Effect of Agavins and Agave Syrup Use in the Formulation of a Synbiotic Gelatin Gummy with Microcapsules of Saccharomyces Boulardii" Gels 10, no. 5: 299. https://doi.org/10.3390/gels10050299