Design and Properties of Novel Hydrophobic Natural Tea Saponin and Its Organogels
Abstract
:1. Introduction
2. Results and Discussion
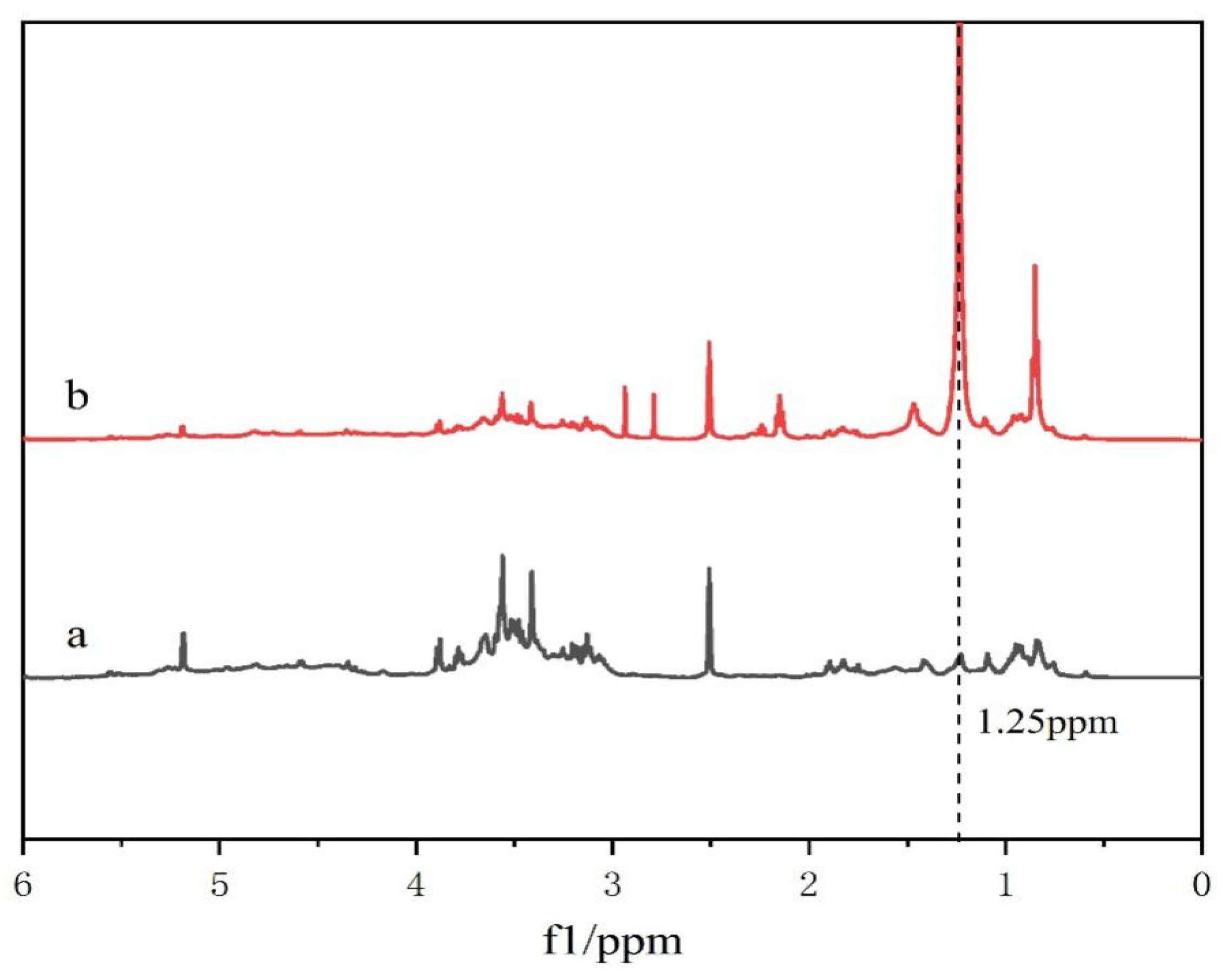
2.1. DC-TS Structure Analysis
2.2. Gelation Studies
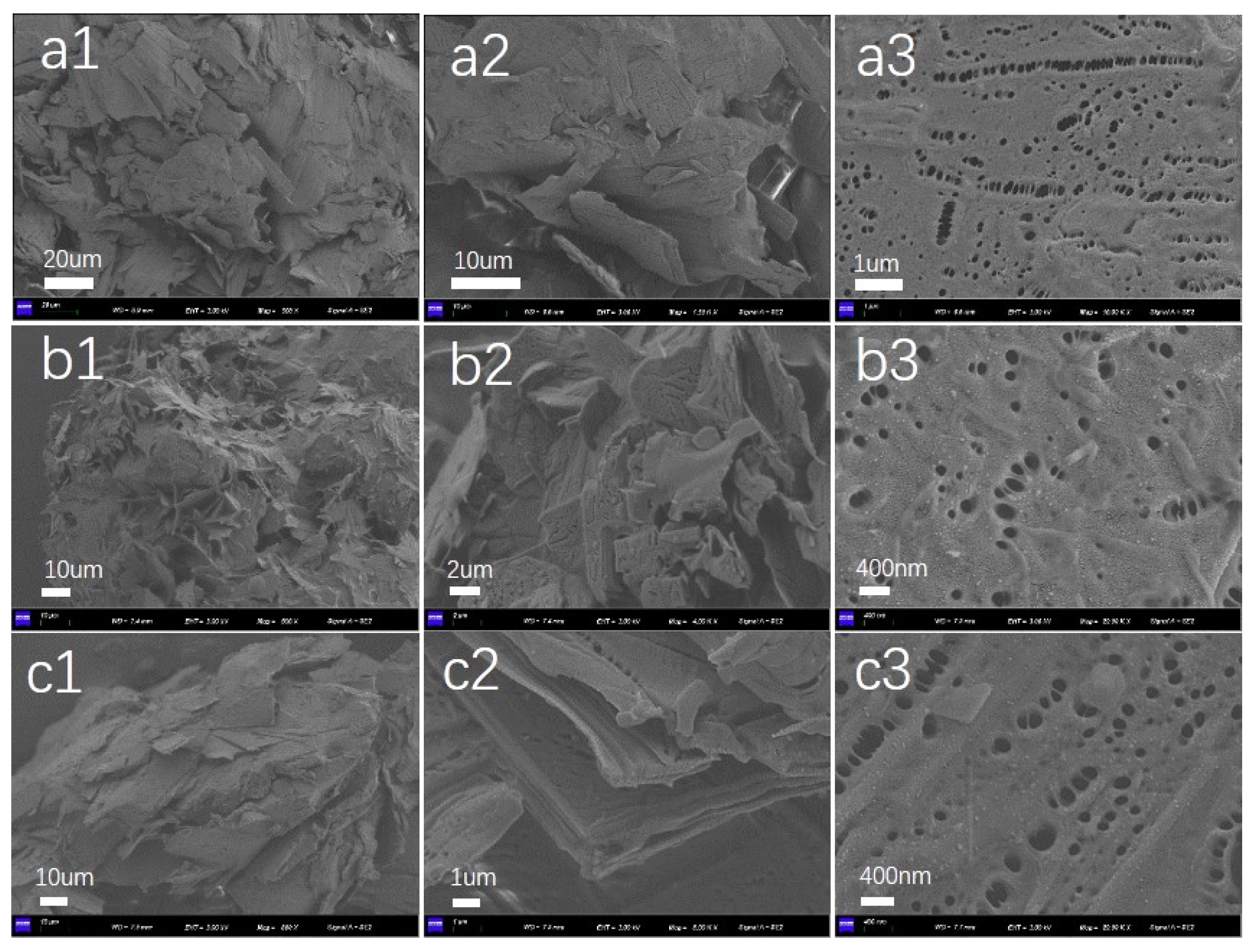
2.3. Morphological Characterization
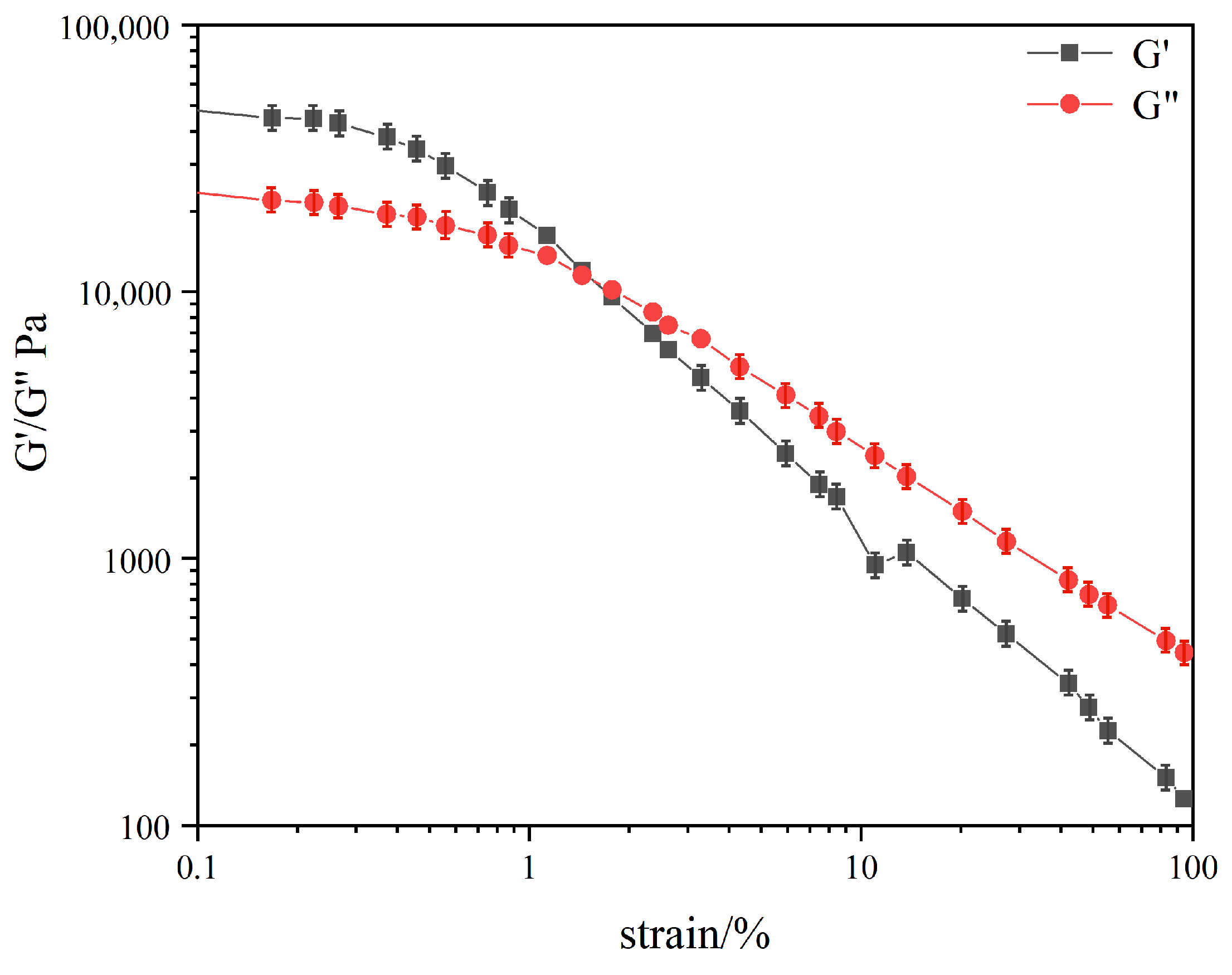
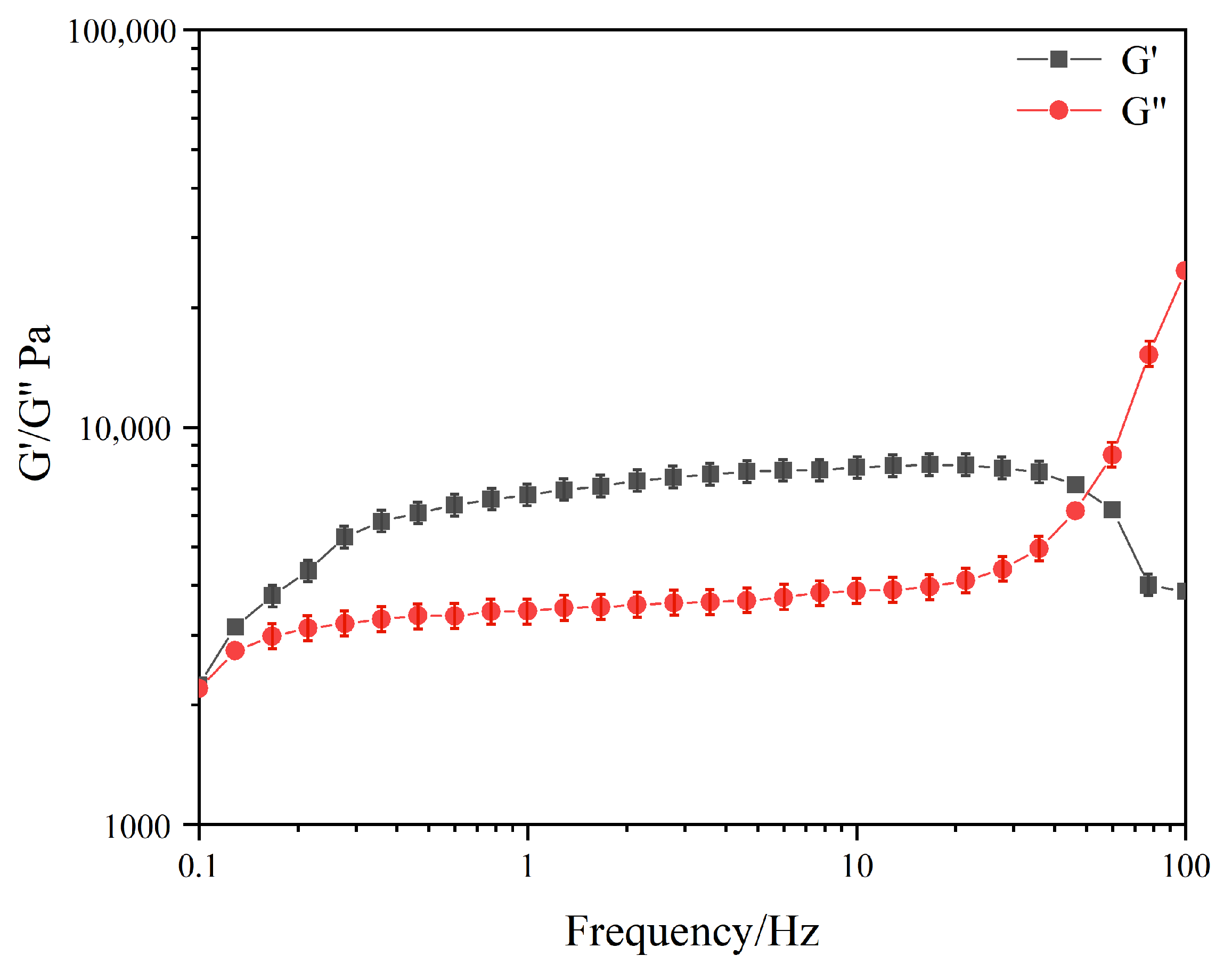
2.4. Rheological Studies
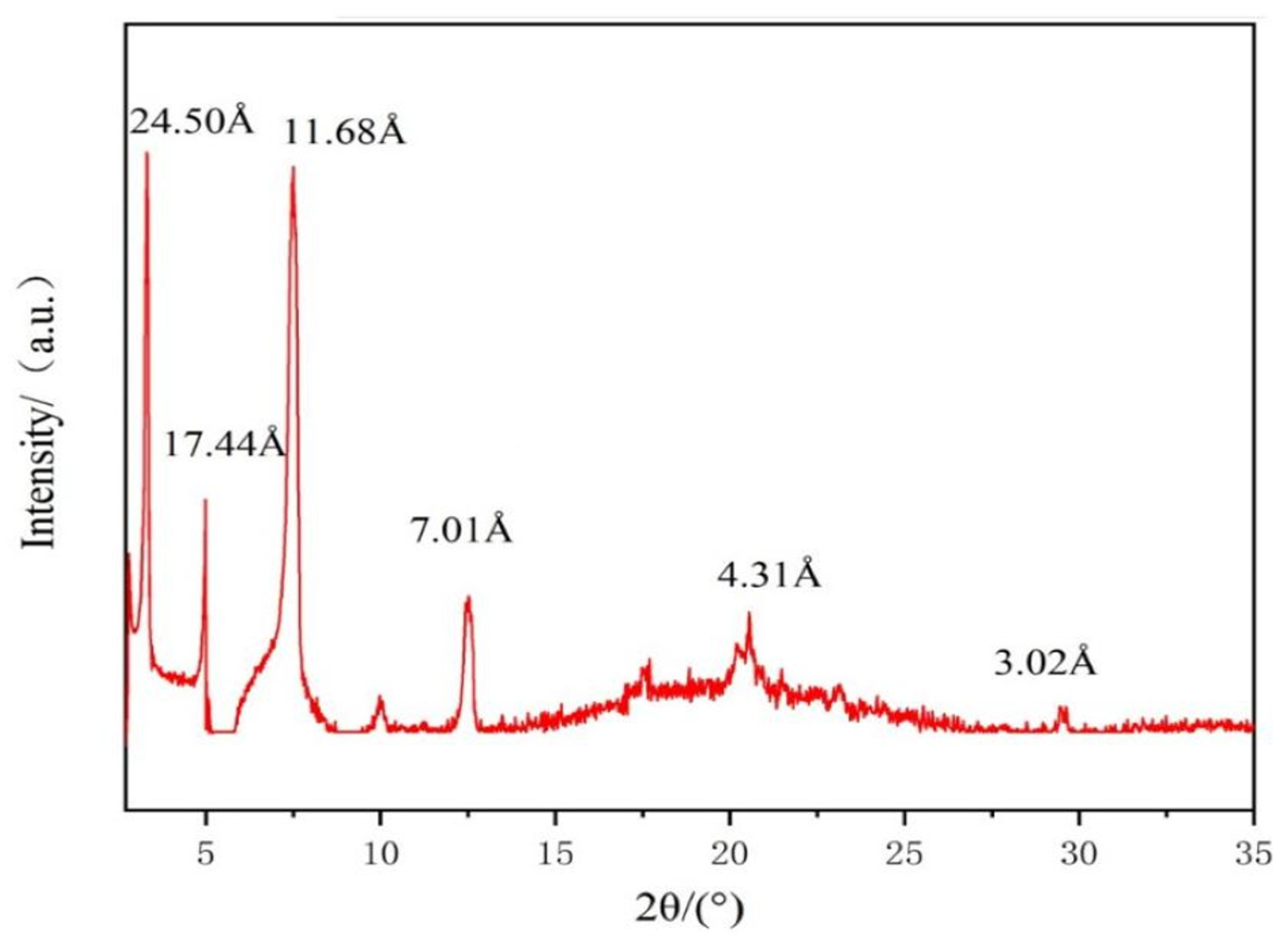
2.5. X-ray Diffraction (XRD) Studies
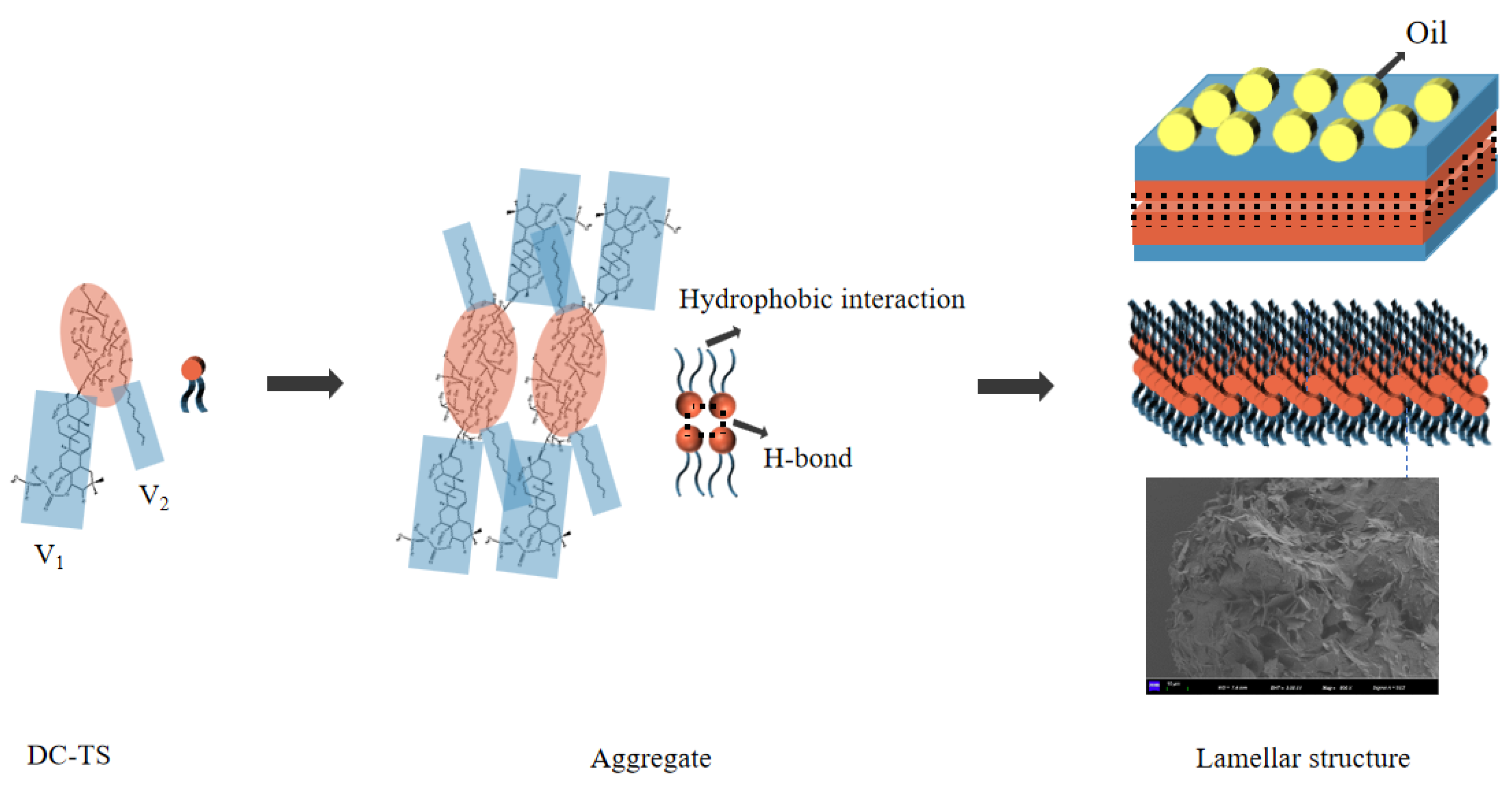
2.6. Investigation of the Driving Forces
2.7. Self-Assembly Process of DC-TS in Organic Solvents
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Dodecyl Chloride–Tea Saponin (DC-TS)
4.3. Preparation of DC-TS Gel
4.4. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, X.; Li, N.; Zhu, H.; Wang, D.; Yang, C.; Zhang, Y. Plant Resources, Chemical Constituents, and Bioactivities of Tea Plants from the Genus Camellia Section Thea. J. Agric. Food Chem. 2019, 67, 5318. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Dias, M.; Barreiro, M.; Pinho, S. Saponins as Natural Emulsifiers for Nanoemulsions. J. Agric. Food Chem. 2022, 70, 6573. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, Y. Tea Saponins: Effective Natural Surfactants Beneficial for Soil Remediation, from Preparation to Application. RSC Adv. 2018, 8, 24312. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yang, M.; Li, J.; Chen, X.; Jiang, J.; Han, C. A Novel Bola-Type Rosin-Based Functional Surfactant and Its Synergistic Effect with Natural Surfactant Saponin. J. Surfactants Deterg. 2017, 20, 1205. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Huang, D.; Li, C.; Xie, M.; Wan, Y. Antimicrobial Activity of Saponin-rich Fraction From Camellia Oleifera Cake and Its Effect on Cell Viability of Mouse Macrophage RAW 264.7. J. Sci. Food Agric. 2012, 92, 2443. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, D.; Liberek, B.; Myszka, H. Synthesis, Modification and Biological Activity of Diosgenyl β-d-Glycosaminosides: An Overview. Molecules 2020, 25, 5433. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Deng, Z.; Wang, T.; Yang, W.; Wang, K. Synthesis of Natural Tea-Saponin-Based Succinic Acid Sulfonate as Anionic Foaming Agent. J. Surfactants Deterg. 2018, 21, 303. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Wu, Z.; Zeng, G.; Shao, B.; Liu, Y.; Jiang, Y.; Zhong, H.; Liu, Y. Fabrication of the Tea Saponin Functionalized Reduced Graphene Oxide for Fast Adsorptive Removal of Cd(II) from Water. Appl. Phys. A 2018, 124, 398. [Google Scholar] [CrossRef]
- Marciani, D. Effects of N-acylation on the Immune Adjuvanticity of Analogs of the Quillaja Saponins Derivative GPI-0100. Vaccine 2022, 40, 4169. [Google Scholar] [CrossRef]
- Huo, G.; Liu, C.; Hui, Y.; Chen, X.; Xiao, D. Synthesis and Structure-Activity Relationship of Oleanolic Mono- or Di-glycosides against Magnaporthe oryzae. Genet. Mol. Res. 2016, 15, 2153. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, X.; Liu, S. Efficient Improvement of Surface Activity of Tea Saponin Through Gemini-like Modification by Straightforward Esterification. Food Chem. 2015, 171, 272. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Xu, G.; Xin, X. Effect of Bile Salts on Self-Assembly and Construction of Micro-/nanomaterials. Acta Phys.-Chim. Sin. 2019, 35, 684. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Morelli, G.; Accardo, A. Self-Supporting Hydrogels Based on Fmoc-Derivatized Cationic Hexapeptides for Potential Biomedical Applications. Biomedicines 2021, 9, 678. [Google Scholar] [CrossRef]
- Roza, N.; Afshin, J.; Anousheh, S.; Chew, K.; Lay, C.; Show, P.; Hoda, J.; Berenjian, A. Hydrothermally Extraction of Saponin from Acanthophyllum Glandulosum Root—Physico-chemical Characteristics and Antibacterial Activity Evaluation. Biotechnol. Rep. 2020, 27, e00507. [Google Scholar] [CrossRef]
- Yan, H.; Ni, W.; Yu, L.; Xiao, L.; Ji, Y.; Liu, H. Parisvaniosides A–E, Five New Steroidal Saponins from Paris Vaniotii. Steroids 2022, 177, 108949. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent Advances in Separation and Analysis of Saponins in Natural Products. Separations 2022, 9, 163. [Google Scholar] [CrossRef]
- Wang, C.; Chao, Z.; Sun, W.; Wu, X.; Ito, Y. Isolation of Five Glycosides from the Barks of Ilex Rotunda by High-Speed Counter-Current Chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2363. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Peng, J.; Yan, N.; Yu, H.; Zhang, S.; Liu, K.; Fang, Y. Simple Design But Marvelous Performances: Molecular Gels of Superior Strength and Self-healing Properties. Soft Matter 2013, 9, 1091. [Google Scholar] [CrossRef]
- Buerkle, L.; Li, Z.; Jamieson, A.; Rowan, S. Tailoring the Properties of Guanosine-Based Supramolecular Hydrogels. Langmuir 2009, 25, 8833. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Dong, Y.; Gu, W.; Liu, T.; Xing, X.; Song, J.; Wang, M.; Han, C. Molecular Design, Supramolecular Assembly, and Excellent Dye Adsorption Capacity of Natural Rigid Dehydroabietic Acid-Tailored Amide Organogelators. Langmuir 2022, 38, 8918. [Google Scholar] [CrossRef]
- Guo, X.; Yao, R.; Wang, M.; Michael, R.; Hoard, E.; Song, J.; Liang, F.; Liu, L.; Jiang, J.; Han, C. Molecular Design, Gel properties, and Anti-Settling Ability with Natural Rosin-based Sulfonamide Compounds Regulated by Conjugation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132809. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, W.; Xing, X.; Guo, X.; Jiang, J.; Song, J.; Michael, R.; Wang, M.; Han, C. Designed Synthesis of Natural Rigid Dehydroabietylamine-Tailored Symmetric Benzamide Organogels by Amide Bonds and Rigid Rings Coordinated Self-Assembly Strategy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130487. [Google Scholar] [CrossRef]
- Vemula, P.; Li, J.; John, G. Enzyme Catalysis: Tool to Make and Break Amygdalin Hydrogelators from Renewable Resources: A Delivery Model for Hydrophobic Drugs. J. Am. Chem. Soc. 2006, 128, 8932. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharya, S. Phthalate Mediated Hydrogelation of a Pyrene Based system: A Novel Scaffold for Shape-Persistent, Self-Healing Luminescent Soft Material. J. Mater. Chem. A 2014, 2, 17889. [Google Scholar] [CrossRef]
- Li, Z.; Hao, A.; Li, X. β-Cyclodextrin Supramolecular Organogels Induced by Different Carboxylic Acids that Exhibit Diverse Morphologies. J. Mol. Liq. 2014, 196, 52. [Google Scholar] [CrossRef]
- Lupi, F.; Greco, V.; Baldino, N.; Cindio, B.; Fischer, P.; Gabriele, D. The Effects of Intermolecular Interactions on the Physical Properties of Organogels in Edible Oils. J. Colloid Interface Sci. 2016, 483, 154. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Dong, H.; Yin, G.; Xu, Z.; Li, S. A Smart Supramolecular Hydrogel Exhibiting pH-Modulated Viscoelastic Properties. Adv. Funct. Mater. 2007, 17, 1837. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, J.; You, Y.; Park, S. Wholly π-Conjugated Low-Molecular-Weight Organogelator That Displays Triple-Channel Responses to Fluoride Ions. Langmuir 2014, 30, 2842. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Functional Gels Based on Chemically Modified Graphenes. Adv. Mater. 2014, 26, 3992. [Google Scholar] [CrossRef]
- Zhang, M.; Selvakumar, S.; Zhang, X.; Sibi, M.; Weiss, R. Structural and Solubility Parameter Correlations of Gelation Abilities for Dihydroxylated Derivatives of Long-Chain, Naturally Occurring Fatty Acids. Chem.-A Eur. J. 2015, 21, 8530. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Wang, P.; Yao, B.; Sun, J.; Gong, P.; Zhang, Z.; Qian, C.; Lu, R. Nanofibers of Hydrogen-Bonded Two-Component Gel with Closely Connected p- and n-Channels and Photoinduced Electron Transfer. ACS Appl. Mater. Interfaces 2014, 6, 21426. [Google Scholar] [CrossRef] [PubMed]
- Brevé, T.; Filius, M.; Weerdenburg, S.; Griend, S.; Groeneveld, T.; Denkova, A.; Eelkema, R. Light-Sensitive Phenacyl Crosslinked Dextran Hydrogels for Controlled Delivery. Chem.-A Eur. J. 2022, 28, e202103523. [Google Scholar] [CrossRef] [PubMed]





| Solvent | Gel Ability | Kamlet–Taft Model | |||
|---|---|---|---|---|---|
| Solubility | Gel Capacity | α | β | π* | |
| Petroleum ether (PE) | S | G | 0 | 0 | 0.10 |
| Cyclohexane (CH) | S | G | 0 | 0 | 0 |
| N-octane (OT) | S | PG | 0 | 0 | 0.08 |
| N-heptane (HT) | C | G | 0 | 0 | 0.08 |
| Carbon tetrachloride (CT) | S | G | 0 | 0 | 0.28 |
| Toluene (TL) | C | G | 0 | 0.11 | 0.54 |
| Ether (ET) | I | PG | 0 | 0.47 | 0.27 |
| Benzene (BZ) | C | G | 0 | 0.10 | 0.59 |
| Tetrahydrofuran (TD) | C | U | 0 | 0.54 | 0.51 |
| Ethyl acetate (EA) | I | PG | 0 | 0.45 | 0.55 |
| Trichloromethane (TM) | C | U | 0.44 | 0 | 0.58 |
| Acetone (AT) | I | PG | 0.08 | 0.48 | 0.71 |
| DC-TS | Petroleum Ether | Cyclohexane | N-Heptane | Carbon Tetrachloride | Toluene | Benzene | |
|---|---|---|---|---|---|---|---|
| υasCH2 | 2920 | 2919 | 2920 | 2921 | 2919 | 2921 | 2921 |
| υsCH2 | 2851 | 2850 | 2851 | 2850 | 2850 | 2850 | 2850 |
| υC=O | 1719 | 1721 | 1718 | 1717 | 1718 | 1719 | 1717 |
| υC-O-C | 1468 | 1467 | 1469 | 1467 | 1468 | 1467 | 1469 |
| δasCH2 | 1403 | 1406 | 1400 | 1407 | 1404 | 1407 | 1412 |
| δsCH2 | 1082 | 1070 | 1074 | 1074 | 1079 | 1072 | 1072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yan, L.; Guo, X.; Xing, X.; Liang, F.; Han, C.; Liu, L. Design and Properties of Novel Hydrophobic Natural Tea Saponin and Its Organogels. Gels 2024, 10, 225. https://doi.org/10.3390/gels10040225
Wang M, Yan L, Guo X, Xing X, Liang F, Han C, Liu L. Design and Properties of Novel Hydrophobic Natural Tea Saponin and Its Organogels. Gels. 2024; 10(4):225. https://doi.org/10.3390/gels10040225
Chicago/Turabian StyleWang, Maogong, Liuxin Yan, Xuying Guo, Xinwei Xing, Fengqian Liang, Chunrui Han, and Liujun Liu. 2024. "Design and Properties of Novel Hydrophobic Natural Tea Saponin and Its Organogels" Gels 10, no. 4: 225. https://doi.org/10.3390/gels10040225





