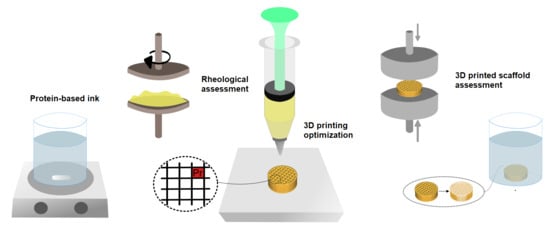
Optimization of Ink Composition and 3D Printing Process to Develop Soy Protein-Based Scaffolds
Abstract
:1. Introduction
2. Results
2.1. Rheological Behavior of 3D Printing Inks
2.2. Shape Fidelity of 3D Printed Scaffolds
2.3. Mechanical Properties of 3D Printed Scaffolds
2.4. Water Uptake of 3D Printed Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Ink Preparation
4.3. Rheological Evaluation
4.4. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
4.5. 3D Printing
4.6. Mechanical Properties
4.7. Water Uptake (WU)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic Hydrogels Designed for Cartilage Tissue Engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.C.; Oliveira, A.S.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-Based Hydrogels Loaded with Diclofenac for Cartilage Replacement. Gels 2022, 8, 143. [Google Scholar] [CrossRef]
- Eze, O.F.; Chatzifragkou, A.; Charalampopoulos, D. Properties of Protein Isolates Extracted by Ultrasonication from Soybean Residue (Okara). Food Chem. 2022, 368, 130837. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy Protein Isolate Supplemented Silk Fibroin Nanofibers for Skin Tissue Regeneration: Fabrication and Characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Funct. Mater. 2020, 30, 1907071. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Dong, W.; Liu, S.; Zhang, H.; Gu, Y. Alginate/Gelatin-Based Hydrogel with Soy Protein/Peptide Powder for 3D Printing Tissue-Engineering Scaffolds to Promote Angiogenesis. Macromol. Biosci. 2022, 22, 2100413. [Google Scholar] [CrossRef] [PubMed]
- Heras, K.L.; Santos-Vizcaino, E.; Garrido, T.; Gutierrez, F.B.; Aguirre, J.J.; de la Caba, K.; Guerrero, P.; Igartua, M.; Hernandez, R.M. Soy Protein and Chitin Sponge-like Scaffolds: From Natural by-Products to Cell Delivery Systems for Biomedical Applications. Green Chem. 2020, 22, 3445–3460. [Google Scholar] [CrossRef]
- Tansaz, S.; Boccaccini, A.R. Biomedical Applications of Soy Protein: A Brief Overview. J. Biomed. Mater. Res. Part A 2016, 104, 553–569. [Google Scholar] [CrossRef]
- Las Heras, K.; Garcia-Orue, I.; Aguirre, J.J.; de la Caba, K.; Guerrero, P.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Soy Protein/β-Chitin Sponge-like Scaffolds Laden with Human Mesenchymal Stromal Cells from Hair Follicle or Adipose Tissue Promote Diabetic Chronic Wound Healing. Biomater. Adv. 2023, 155, 213682. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, G.; Liu, X.; Liu, H.; He, Y.; Zhu, D. Morphological and Structural Changes in Thermally-Induced Soybean Protein Isolate Xerogels Modulated by Soybean Polysaccharide Concentration. Food Hydrocoll. 2022, 133, 107967. [Google Scholar] [CrossRef]
- Durand, D.; Christophe Gimel, J.; Nicolai, T. Aggregation, Gelation and Phase Separation of Heat Denatured Globular Proteins. Phys. A Stat. Mech. Its Appl. 2002, 304, 253–265. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-Induced Gelation of Plant Globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Wang, J.; Burton Navicha, W.; Na, X.; Ma, W.; Xu, X.; Wu, C.; Du, M. Preheat-Induced Soy Protein Particles with Tunable Heat Stability. Food Chem. 2021, 336, 127624. [Google Scholar] [CrossRef]
- Ma, W.; Wang, T.; Wang, J.; Wu, D.; Wu, C.; Du, M. Enhancing the Thermal Stability of Soy Proteins by Preheat Treatment at Lower Protein Concentration. Food Chem. 2020, 306, 125593. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Siu, W.K.; Sagis, L.M.C. Linear and Non-Linear Rheology of Heat-Set Soy Protein Gels: Effects of Selective Proteolysis of β-Conglycinin and Glycinin. Food Hydrocoll. 2021, 120, 106962. [Google Scholar] [CrossRef]
- Guerrero, P.; Garrido, T.; Garcia-Orue, I.; Santos-Vizcaino, E.; Igartua, M.; Hernandez, R.M.; de la Caba, K. Characterization of Bio-Inspired Electro-Conductive Soy Protein Films. Polymers 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Carranza, T.; Zalba-Balda, M.; Baraibar, M.J.B.; Guerrero, P.; de la Caba, K. Effect of Sterilization Processes on Alginate/Gelatin Inks for Three-Dimensional Printing. Int. J. Bioprint. 2022, 9, 645. [Google Scholar] [CrossRef]
- Babaei, J.; Mohammadian, M.; Madadlou, A. Gelatin as Texture Modifier and Porogen in Egg White Hydrogel. Food Chem. 2019, 270, 189–195. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of Soy Protein Isolate and Hydrocolloids Based Mixtures as Promising Food Material in 3D Food Printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Tansaz, S.; Singh, R.; Cicha, I.; Boccaccini, A.R. Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility. Polymers 2018, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, C.; Guo, Y.; Liu, X.; Zhu, F.; Liu, Z.; Liu, X.; Yang, F. Rheological & 3D Printing Properties of Potato Starch Composite Gels. J. Food Eng. 2022, 313, 110756. [Google Scholar] [CrossRef]
- Alam, M.R.; Shahid, M.A.; Alimuzzaman, S.; Khan, A.N. Sources, Extractions and Applications of Bio-Maker Collagen–A Review. Biomed. Eng. Adv. 2022, 4, 100064. [Google Scholar] [CrossRef]
- Carranza, T.; Uranga, J.; Irastorza, A.; Izeta, A.; Guerrero, P.; de la Caba, K. Combination of 3D Printing and Electrospinning to Develop Chitin/Gelatin/PVA Scaffolds. Int. J. Bioprint. 2023, 9, 701. [Google Scholar] [CrossRef]
- Cheng, Y.; Fu, Y.; Ma, L.; Yap, P.L.; Losic, D.; Wang, H.; Zhang, Y. Rheology of Edible Food Inks from 2D/3D/4D Printing, and Its Role in Future 5D/6D Printing. Food Hydrocoll. 2022, 132, 107855. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Network: Hannover, Germany, 2014; ISBN 978-3-7486-0036-7. [Google Scholar]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic Interaction between Proteins and Polysaccharides: Physicochemical Aspects and Applications in Emulsion Stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Xu, K.; Wu, C.; Fan, G.; Kou, X.; Li, X.; Li, T.; Dou, J.; Zhou, Y. Rheological Properties, Gel Properties and 3D Printing Performance of Soy Protein Isolate Gel Inks Added with Different Types of Apricot Polysaccharides. Int. J. Biol. Macromol. 2023, 242, 124624. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, C.; Du, Z.; Zou, W.; Li, H. Structure and Properties of Poly (Vinyl Alcohol)/Soy Protein Isolate Blend Film Fabricated Through Melt Processing. J. Polym. Environ. 2015, 23, 183–189. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effect of Preheating-Induced Denaturation during Protein Production on the Structure and Gelling Properties of Soybean Proteins. Food Hydrocoll. 2020, 105, 105846. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of Pre-Heating Soybean Protein Isolate and Transglutaminase Treatments on the Properties of Egg-Soybean Protein Isolate Composite Gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Tailoring Soy Protein Film Properties by Selecting Casting or Compression as Processing Methods. Eur. Polym. J. 2016, 85, 499–507. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Dong, X.; Prakash, S. Feasibility Study of Hydrocolloid Incorporated 3D Printed Pork as Dysphagia Food. Food Hydrocoll. 2020, 107, 105940. [Google Scholar] [CrossRef]
- Avallone, P.R.; Raccone, E.; Costanzo, S.; Delmonte, M.; Sarrica, A.; Pasquino, R.; Grizzuti, N. Gelation Kinetics of Aqueous Gelatin Solutions in Isothermal Conditions via Rheological Tools. Food Hydrocoll. 2021, 111, 106248. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z.; Huang, Y.; Niu, L.; Miao, J.; Lai, K. Effects of Acidulants on the Rheological Properties of Gelatin Extracted from the Skin of Tilapia (Oreochromis Mossambicus). Foods 2022, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Fu, Y.; He, J. Preparation and Physical Properties of Soy Protein Isolate and Gelatin Composite Films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved Texture Analysis for Hydrogel Characterization: Gel Cohesiveness, Adhesiveness, and Hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Janarthanan, G.; Shin, H.S.; Kim, I.-G.; Ji, P.; Chung, E.-J.; Lee, C.; Noh, I. Self-Crosslinking Hyaluronic Acid–Carboxymethylcellulose Hydrogel Enhances Multilayered 3D-Printed Construct Shape Integrity and Mechanical Stability for Soft Tissue Engineering. Biofabrication 2020, 12, 045026. [Google Scholar] [CrossRef] [PubMed]
- De Roo, C.; Tilleman, K.; Vercruysse, C.; Declercq, H.; T’Sjoen, G.; Weyers, S.; De Sutter, P. Texture Profile Analysis Reveals a Stiffer Ovarian Cortex after Testosterone Therapy: A Pilot Study. J. Assist. Reprod. Genet. 2019, 36, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.K.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, Q.; Zhao, Y.; Zhao, D.; Zhao, X.; Jiang, L.; Zhang, Y.; Wu, F.; Sui, X. Improving Gel Properties of Soy Protein Isolate through Alkaline pH-Shifting, Mild Heat Treatment, and TGase Cross-Linking. Food Hydrocoll. 2023, 144, 108924. [Google Scholar] [CrossRef]
- Nishinari, K.; Turcanu, M.; Nakauma, M.; Fang, Y. Role of Fluid Cohesiveness in Safe Swallowing. NPJ Sci. Food 2019, 3, 5. [Google Scholar] [CrossRef]
- Karami, P.; Stampoultzis, T.; Guo, Y.; Pioletti, D.P. A Guide to Preclinical Evaluation of Hydrogel-Based Devices for Treatment of Cartilage Lesions. Acta Biomater. 2023, 158, 12–31. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Hydrogel Based Materials: A Progressive Approach towards Advancement in Biomedical Applications. Mater. Today Commun. 2022, 33, 104369. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, J.; Zhu, M.; Yang, H.; Liu, J.; Liu, Y. Study of Physicochemical and Gelation Properties of Fish Gelatin from Different Sources. Appl. Sci. 2023, 13, 5337. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Aguilar, J.M.; Bengoechea, C.; López-Castejón, M.L.; Guerrero, A. Rheology and Water Absorption Properties of Alginate–Soy Protein Composites. Polymers 2021, 13, 1807. [Google Scholar] [CrossRef]
- Calafel, I.; Aguirresarobe, R.H.; Peñas, M.I.; Santamaria, A.; Tierno, M.; Conde, J.I.; Pascual, B. Searching for Rheological Conditions for FFF 3D Printing with PVC Based Flexible Compounds. Materials 2020, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pizzetti, F.; Maspes, A.; Rossetti, A.; Rossi, F. The Addition of Hyaluronic Acid in Chemical Hydrogels Can Tune the Physical Properties and Degradability. Eur. Polym. J. 2021, 161, 110843. [Google Scholar] [CrossRef]






| Sample | Hardness (g) | Cohesiveness |
|---|---|---|
| SPI25PVA-H | 2923 ± 139 a | 0.704 ± 0.184 a |
| SPI25PVA3SA | 2027 ± 51 ab | 0.843 ± 0.002 a |
| SPI25PVA3SA-H | 2170 ± 61 b | 0.822 ± 0.003 a |
| SPI25PVA2GEL | 3001 ± 121 b | 0.830 ± 0.003 ab |
| SPI25PVA2GEL-H | 3008 ± 168 b | 0.830 ± 0.001 ab |
| SPI25PVA3GEL | 3402 ± 70 c | 0.700 ± 0.085 ab |
| SPI25PVA3GEL-H | 3761 ± 361 c | 0.564 ± 0.050 b |
| Ink designation | SPI (w/v%) | PVA (wt%) | SA (wt%) | GEL (wt%) |
|---|---|---|---|---|
| SPI20PVA | 25 | 20 | 0 | 0 |
| SPI25PVA | 25 | 25 | 0 | 0 |
| SPI30PVA | 25 | 30 | 0 | 0 |
| SPI25PVA1SA | 25 | 25 | 1 | 0 |
| SPI25PVA2SA | 25 | 25 | 2 | 0 |
| SPI25PVA3SA | 25 | 25 | 3 | 0 |
| SPI25PVA1GEL | 25 | 25 | 0 | 1 |
| SPI25PVA2GEL | 25 | 25 | 0 | 2 |
| SPI25PVA3GEL | 25 | 25 | 0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza, T.; Tejo-Otero, A.; Bengoechea, C.; Guerrero, P.; de la Caba, K. Optimization of Ink Composition and 3D Printing Process to Develop Soy Protein-Based Scaffolds. Gels 2024, 10, 223. https://doi.org/10.3390/gels10040223
Carranza T, Tejo-Otero A, Bengoechea C, Guerrero P, de la Caba K. Optimization of Ink Composition and 3D Printing Process to Develop Soy Protein-Based Scaffolds. Gels. 2024; 10(4):223. https://doi.org/10.3390/gels10040223
Chicago/Turabian StyleCarranza, Teresa, Aitor Tejo-Otero, Carlos Bengoechea, Pedro Guerrero, and Koro de la Caba. 2024. "Optimization of Ink Composition and 3D Printing Process to Develop Soy Protein-Based Scaffolds" Gels 10, no. 4: 223. https://doi.org/10.3390/gels10040223