Biomimetic Gradient Hydrogels with High Toughness and Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
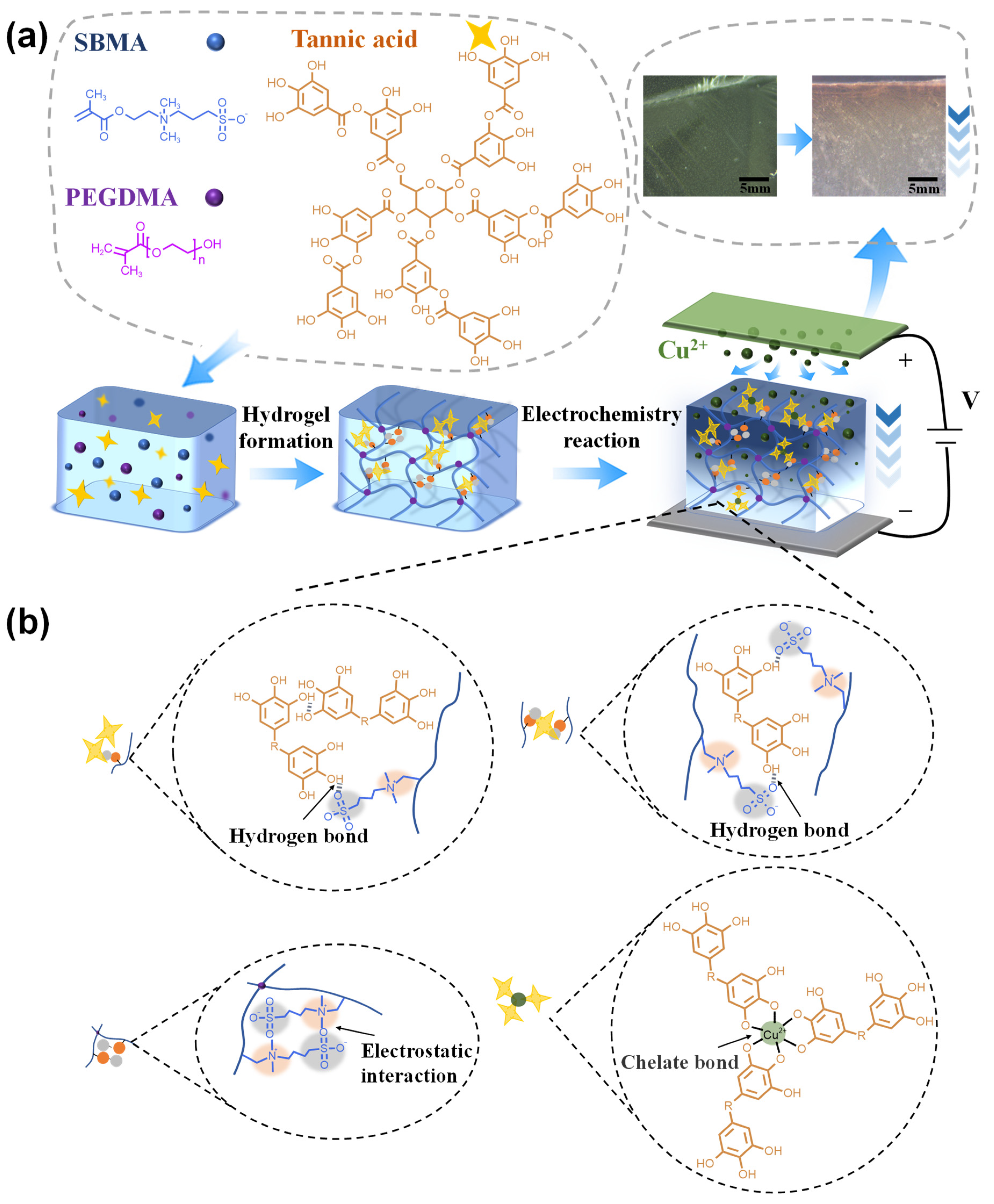
2.1. Fabrication of Cu Gradient Hydrogel
2.2. Mechanical Properties
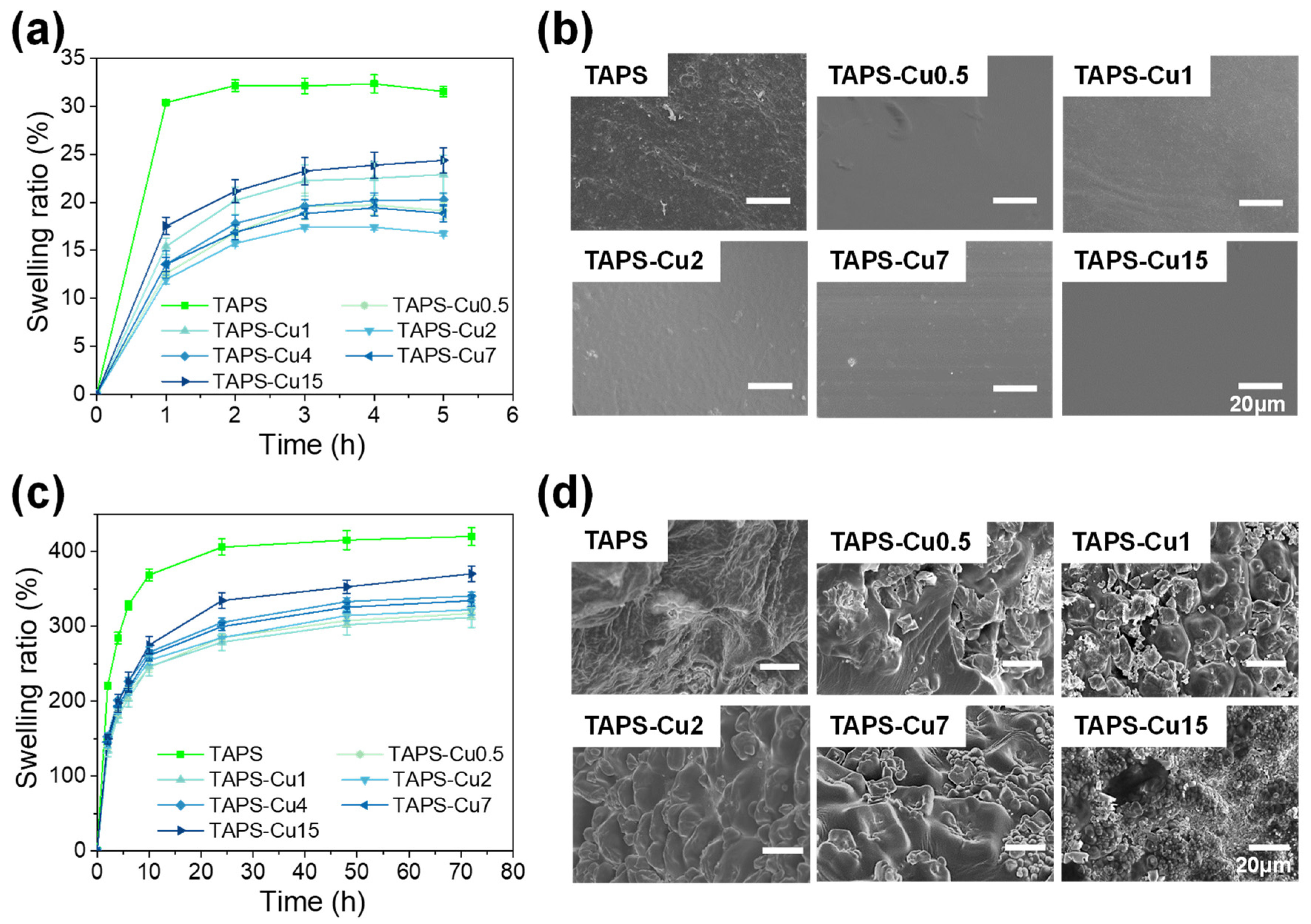
2.3. Swelling Property
2.4. Gradient Cu Cross-Linking Structure and Cu Release from the Hydrogel
2.5. Antibacterial Activity
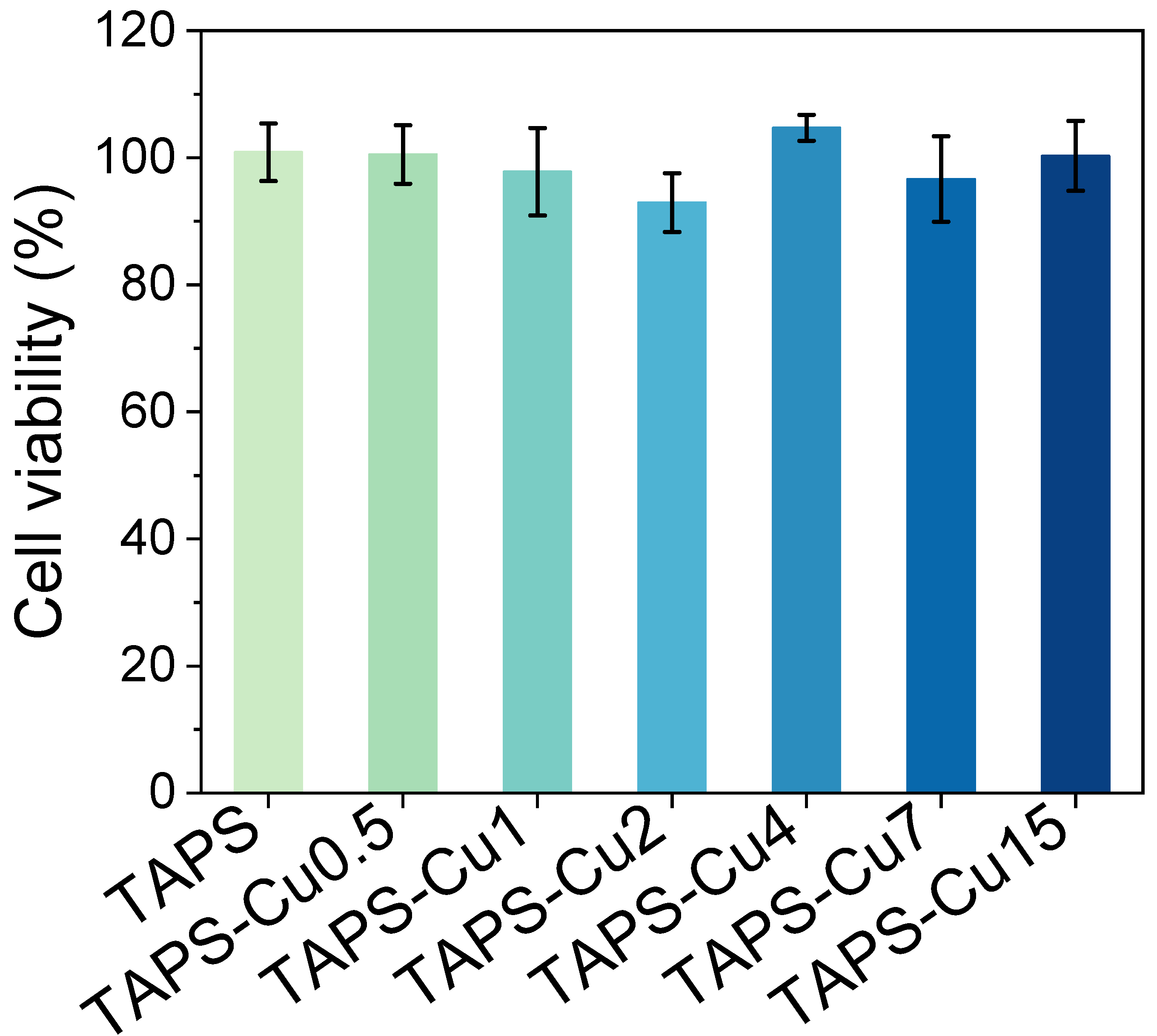
2.6. Cytotoxicity Assay
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Hydrogel Preparation
4.3. Mechanical Properties and Swelling Behavior
4.4. Determination of Cu Content and Cu Release from Hydrogel
4.5. Antibacterial Test
4.6. Cytotoxicity Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Z. Tissue-Resident Memory T Cells and Their Biological Characteristics in the Recurrence of Inflammatory Skin Disorders. Cell. Mol. Immunol. 2020, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mayandi, V.; Wen Choong, A.C.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.H.U.T.; et al. Multifunctional Antimicrobial Nanofiber Dressings Containing ε-Polylysine for the Eradication of Bacterial Bioburden and Promotion of Wound Healing in Critically Colonized Wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pan, G.; Zhang, H.; Guo, B. Hydrogel Adhesives for Generalized Wound Treatment: Design and Applications. J. Polym. Sci. 2022, 60, 1328–1359. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the Molecule Structures to Achieve Functional Advantages of Hydrogel Wound Dressings: Advances and Strategies. Compos. Part B Eng. 2022, 247, 11031. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent Trends on Burn Wound Care: Hydrogel Dressings and Scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent Progress of Highly Adhesive Hydrogels as Wound Dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, 1901396. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, J.; Hong, Y.; Shen, L. Recent Advances in Design Strategies of Tough Hydrogels. Macromol. Rapid Commun. 2022, 43, 2200075. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gong, J.P. Fabrication of Bioinspired Hydrogels: Challenges and Opportunities. Macromolecules 2020, 53, 2769–2782. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong Tough Hydrogels via the Synergy of Freeze-Casting and Salting Out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wei, F.; Hu, J. Muscle Contraction-Inspired Tough Hydrogels. ACS Appl. Mater. Interfaces 2023, 15, 8462–8470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhang, X.N.; Song, Y.; Zhao, Y.; Chen, L.; Su, F.; Li, L.; Wu, Z.L.; Zheng, Q. Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally Graded Materials: A Review of Fabrication and Properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Quan, H.; Kisailus, D.; Meyers, M.A. Hydration-Induced Reversible Deformation of Biological Materials. Nat. Rev. Mater. 2020, 6, 264–283. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- McCaw, B.A.; Stevenson, T.J.; Lancaster, L.T. Epigenetic Responses to Temperature and Climate. Integr. Comp. Biol. 2020, 60, 1469–1480. [Google Scholar] [CrossRef]
- Wijerathne, B.; Liao, T.; Ostrikov, K.; Sun, Z. Bioinspired Robust Mechanical Properties for Advanced Materials. Small Struct. 2022, 3, 2100228. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Dev. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Must, I.; Sinibaldi, E.; Mazzolai, B. A Variable-Stiffness Tendril-like Soft Robot Based on Reversible Osmotic Actuation. Nat. Commun. 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chang, Y.; Yu, Z.; Liang, Y.; Ren, L.; Ren, L. Bionic Intelligent Soft Actuators: High-Strength Gradient Intelligent Hydrogels with Diverse Controllable Deformations and Movements. J. Mater. Chem. B 2020, 8, 9362–9373. [Google Scholar] [CrossRef] [PubMed]
- Vigolo, D.; Ramakrishna, S.N.; deMello, A.J. Facile Tuning of the Mechanical Properties of a Biocompatible Soft Material. Sci. Rep. 2019, 9, 7125. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.F.; Zhang, X.N.; Du, C.; Frank, A.; Schmidt, H.-W.; Zheng, Q.; Wu, Z.L. Photoregulated Gradient Structure and Programmable Mechanical Performances of Tough Hydrogels with a Hydrogen-Bond Network. ACS Appl. Mater. Interfaces 2020, 12, 53376–53384. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent Advances in Natural Polymer-Based Drug Delivery Systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; De Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhang, Y.; Dai, Y.; Xia, F.; Zhang, X. Adhesive and Tough Hydrogels: From Structural Design to Applications. J. Mater. Chem. B 2021, 9, 5954–5966. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Jeon, I. Biomimetic Anisotropic Hydrogels: Advanced Fabrication Strategies, Extraordinary Functionalities, and Broad Applications. Prog. Mater. Sci. 2022, 124, 100870. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Obregón, R.; Shiku, H.; Matsue, T. Facile and Rapid Generation of 3D Chemical Gradients within Hydrogels for High-Throughput Drug Screening Applications. Biosens. Bioelectron. 2014, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, T.; Pan, X. Metal–Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Gu, Q.; Zeng, M.; Huang, Z.; Qiu, H.; Miao, J.; Fang, Y.; Zhao, Y.; Xiao, Y.; Xu, T.; et al. Tannic Acid-Reinforced Zwitterionic Hydrogels with Multi-Functionalities for Diabetic Wound Treatment. J. Mater. Chem. B 2022, 10, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Sanger, M.J.; Greenbowe, T.J. An Analysis of College Chemistry Textbooks As Sources of Misconceptions and Errors in Electrochemistry. J. Chem. Educ. 1999, 76, 853. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering Multifunctional Capsules through the Assembly of Metal–Phenolic Networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xue, J.; Tang, C. MnO2-Coated Graphene/Polypyrrole Hybrids for Enhanced Capacitive Deionization Performance of Cu 2+ Removal. Ind. Eng. Chem. Res. 2022, 61, 3582–3590. [Google Scholar] [CrossRef]
- Fei, J.; Ding, B.; Koh, S.W.; Ge, J.; Wang, X.; Lee, L.; Sun, Z.; Yao, M.; Chen, Y.; Gao, H.; et al. Mechanistic Investigation of Electrostatic Field-Enhanced Water Evaporation. Adv. Sci. 2021, 8, 2100875. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Pan, L.; Zhong, D.; Liu, Y. Porous Electrode Improving Energy Efficiency under Electrode-Normal Magnetic Field in Water Electrolysis. Int. J. Hydrogen Energy 2019, 44, 22780–22786. [Google Scholar] [CrossRef]
- Asoh, T.-A. Electrophoretic Hydrogel Adhesion for Fabrication of Three-Dimensional Materials. Polym. J. 2016, 48, 1095–1101. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1970337. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Yan, F.; Xiang, X.; Tang, Y.; Zhang, L.; Liu, J.; Qiu, L. Determination of Normal Skin Elasticity by Using Real-time Shear Wave Elastography. J. Ultrasound Med. 2018, 37, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Hong, I.; Kim, T.; Lee, E.; Kim, E.; Ryu, J.-K.; Jo, Y.; Koo, J.; Han, S.; et al. Foot Plantar Pressure Measurement System Using Highly Sensitive Crack-Based Sensor. Sensors 2019, 19, 5504. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, C.; Wu, C.; Zhang, W. Gastric Fluid-Induced Double Network Hydrogel with High Swelling Ratio and Long-Term Mechanical Stability. Composites. Part B 2022, 236, 109816. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D Laminar Membranes for Selective Water and Ion Transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Chang, T.; Babu, R.P.; Zhao, W.; Johnson, C.M.; Hedström, P.; Odnevall, I.; Leygraf, C. High-Resolution Microscopical Studies of Contact Killing Mechanisms on Copper-Based Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 49402–49413. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Biomater. Adv. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Z.; Bai, Y.; Chen, Z.; Qin, J.; Feng, F. A Novel Highly Fluorescent S, N, O Co-Doped Carbon Dots for Biosensing and Bioimaging of Copper Ions in Live Cells. RSC Adv. 2018, 8, 42246–42252. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- ISO TDC 9 (1697) DTZS; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.; Peng, L.; Wang, D.; Zhang, Y.; Yan, B.; Xie, J.; Xing, S.; Peng, F.; Liu, X. A Copper-Metal Organic Framework Enhances the Photothermal and Chemodynamic Properties of Polydopamine for Melanoma Therapy. Acta Biomater. 2023, 158, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal–Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]

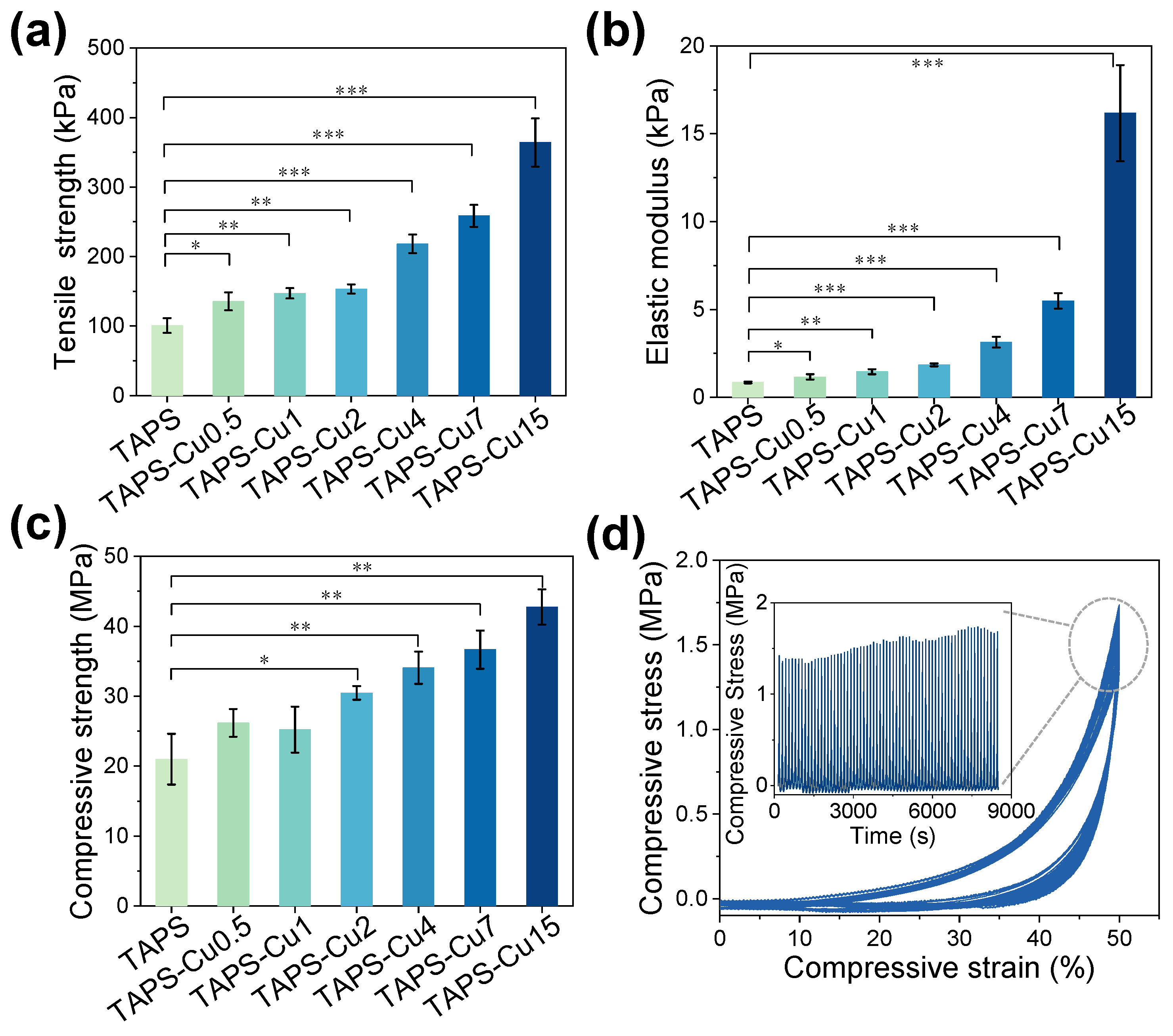

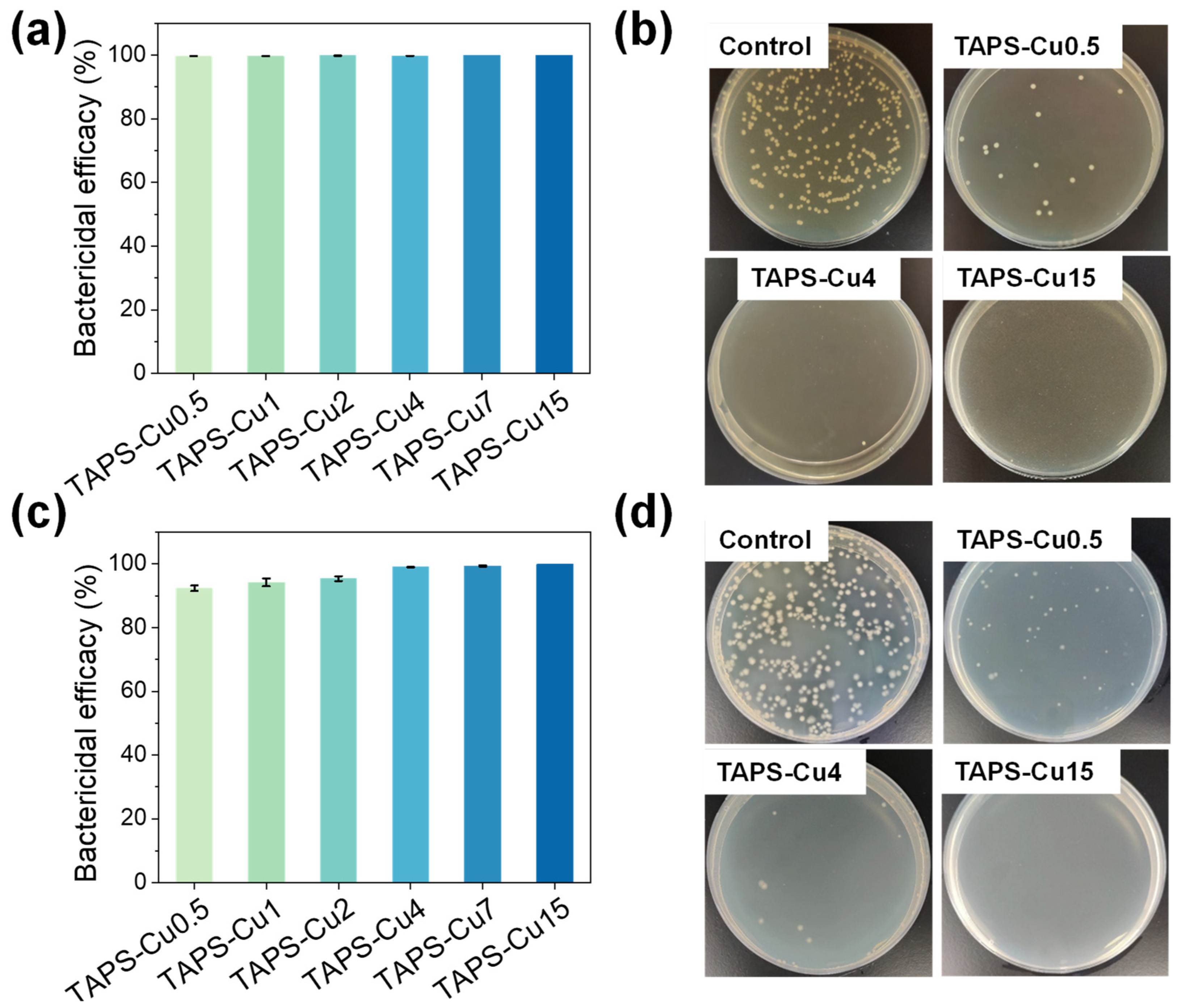
| Hydrogel Name | Electrophoresis Time (min) | Electric Charge (C) | Tensile Strength (kPa) | Elastic Modulus (kPa) | Compressive Strength (MPa) | Bactericidal Efficacy (%) | |
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | ||||||
| TAPS | 0 | 0 | 100.80 ± 10.62 | 0.85 ± 0.05 | 21.00 ± 3.64 | / | / |
| TAPS-Cu0.5 | 0.5 | 0.90 ± 0.08 | 135.54 ± 13.00 | 1.17 ± 0.16 | 26.19 ± 1.99 | 99.66 ± 0.01 | 92.35 ± 0.87 |
| TAPS-Cu1 | 1 | 1.62 ± 0.27 | 147.20 ± 7.44 | 1.46 ± 0.14 | 25.24 ± 3.29 | 99.68 ± 0.01 | 94.21 ± 1.24 |
| TAPS-Cu2 | 2 | 2.94 ± 0.10 | 153.35 ± 6.54 | 1.85 ± 0.08 | 30.48 ± 0.99 | 99.81 ± 0.01 | 95.39 ± 0.73 |
| TAPS-Cu4 | 4 | 3.76 ± 0.44 | 218.28 ± 13.23 | 3.15 ± 0.31 | 34.08 ± 2.30 | 99.77 ± 0.02 | 99.04 ± 0.09 |
| TAPS-Cu7 | 7 | 5.83 ± 0.36 | 258.59 ± 15.94 | 5.49 ± 0.44 | 36.67 ± 2.74 | 99.99 ± 0 | 99.39 ± 0.17 |
| TAPS-Cu15 | 15 | 7.84 ± 0.80 | 364.14 ± 34.56 | 16.1 ± 2.74 | 42.77 ± 2.52 | 100 ± 0 | 99.99 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Huang, Z.; Cen, X.; Zhao, Y.; Xu, F.; Miao, J.; Zhang, Q.; Wang, R. Biomimetic Gradient Hydrogels with High Toughness and Antibacterial Properties. Gels 2024, 10, 6. https://doi.org/10.3390/gels10010006
Zeng M, Huang Z, Cen X, Zhao Y, Xu F, Miao J, Zhang Q, Wang R. Biomimetic Gradient Hydrogels with High Toughness and Antibacterial Properties. Gels. 2024; 10(1):6. https://doi.org/10.3390/gels10010006
Chicago/Turabian StyleZeng, Mingzhu, Zhimao Huang, Xiao Cen, Yinyu Zhao, Fei Xu, Jiru Miao, Quan Zhang, and Rong Wang. 2024. "Biomimetic Gradient Hydrogels with High Toughness and Antibacterial Properties" Gels 10, no. 1: 6. https://doi.org/10.3390/gels10010006





