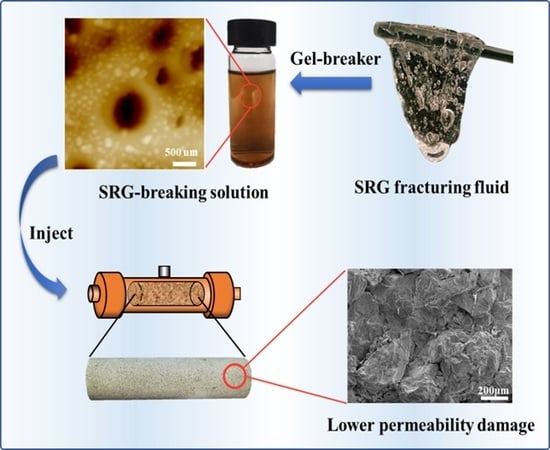
A Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing
Abstract
:1. Introduction
2. Results and Discussion
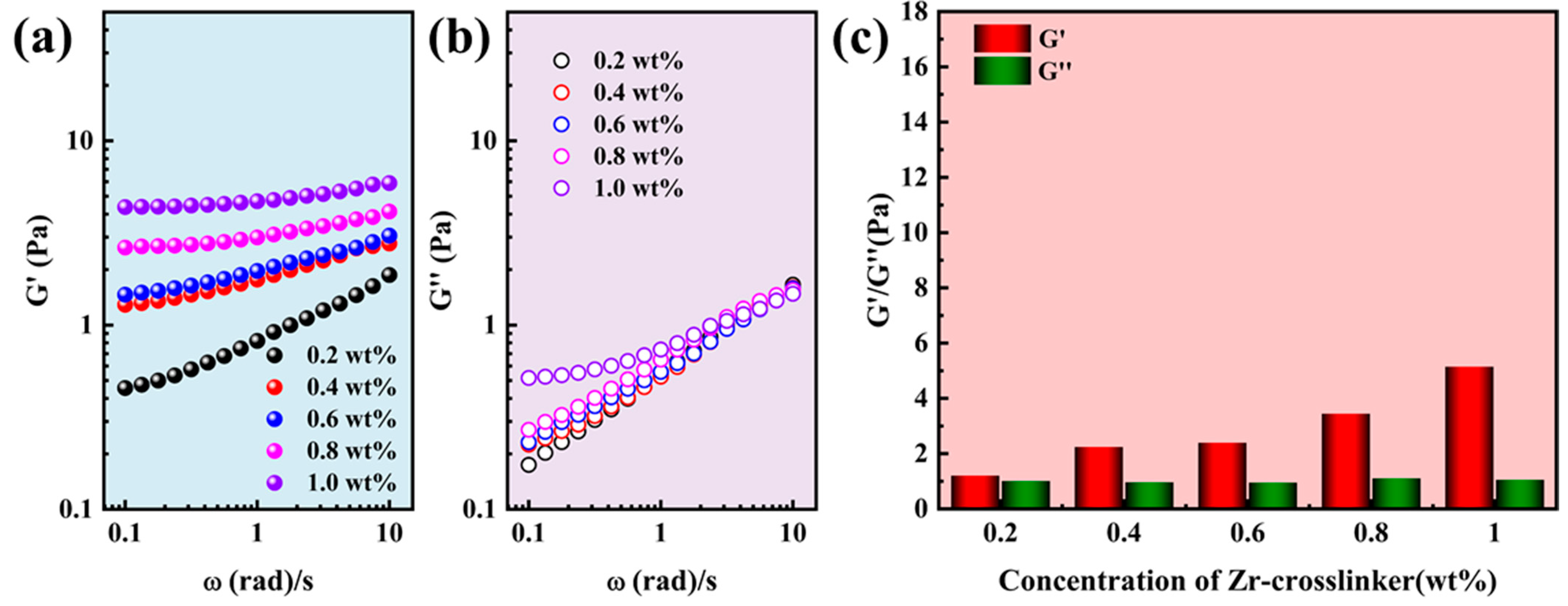
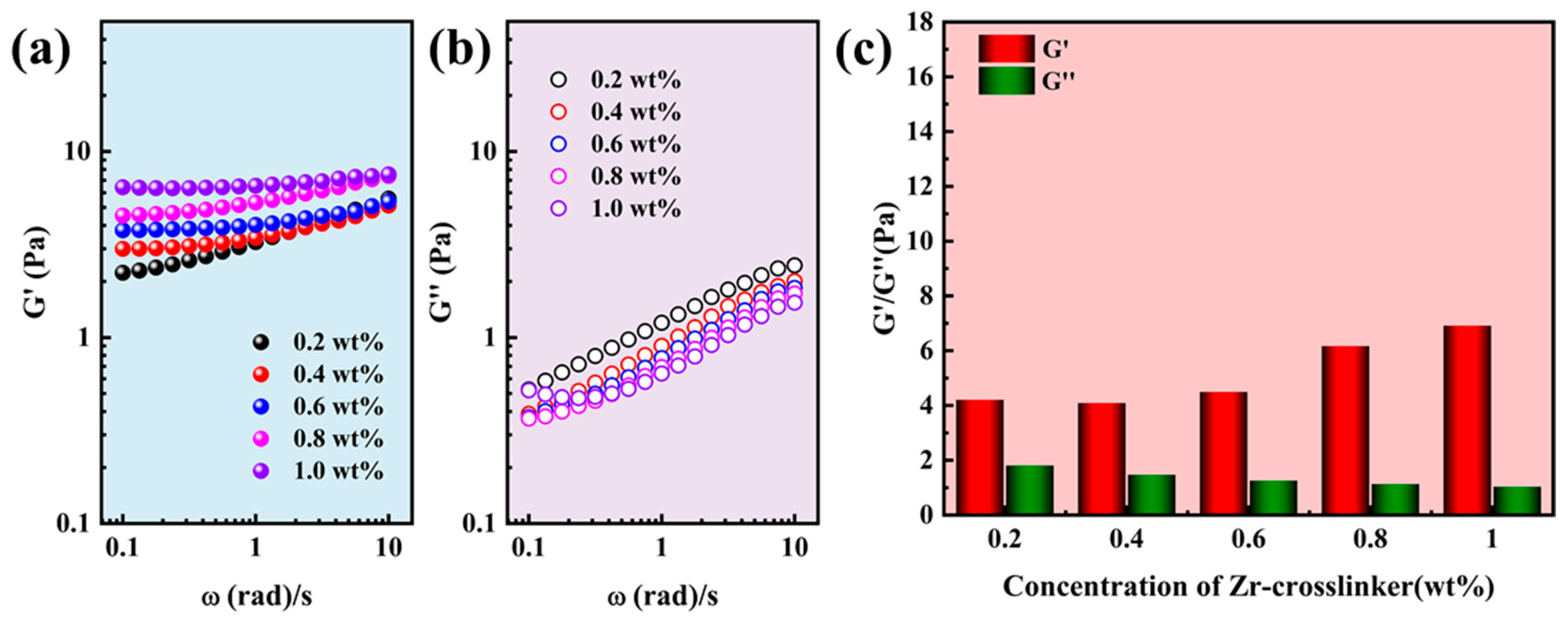
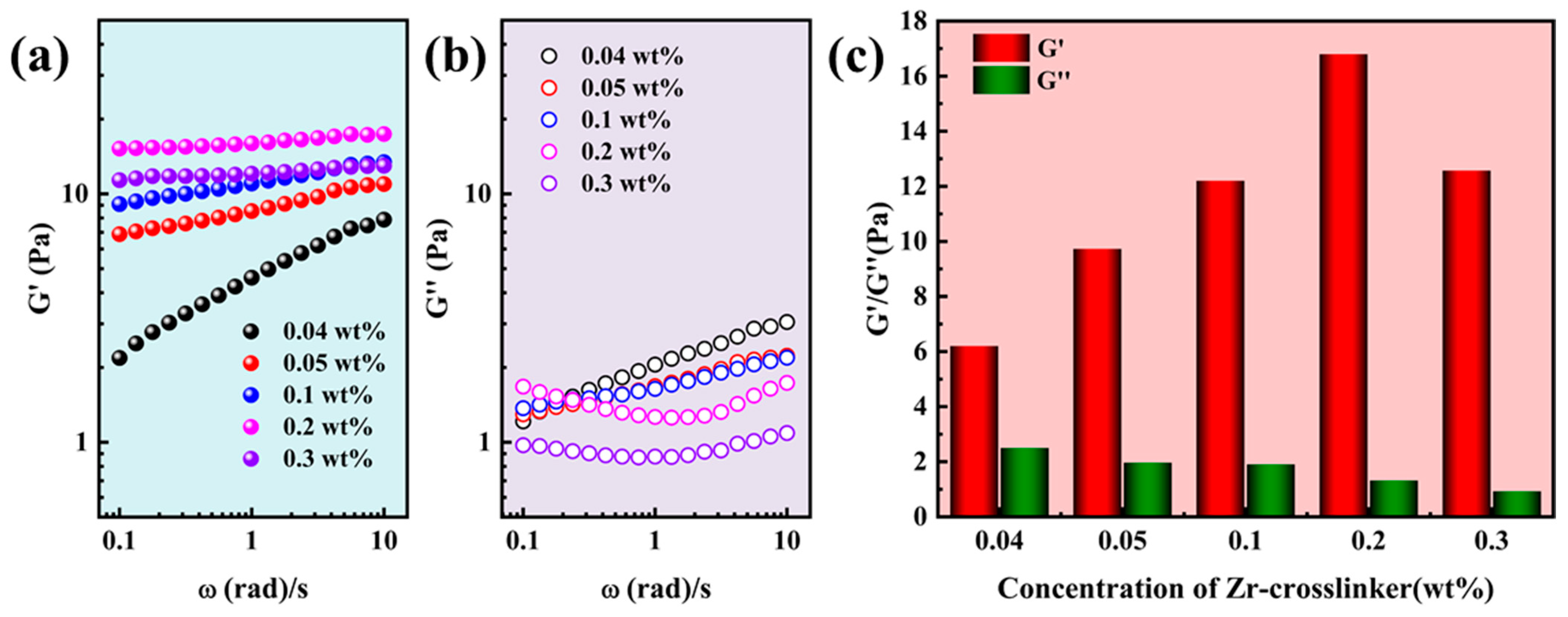
2.1. Rheological Properties of Gel Fracturing Fluid
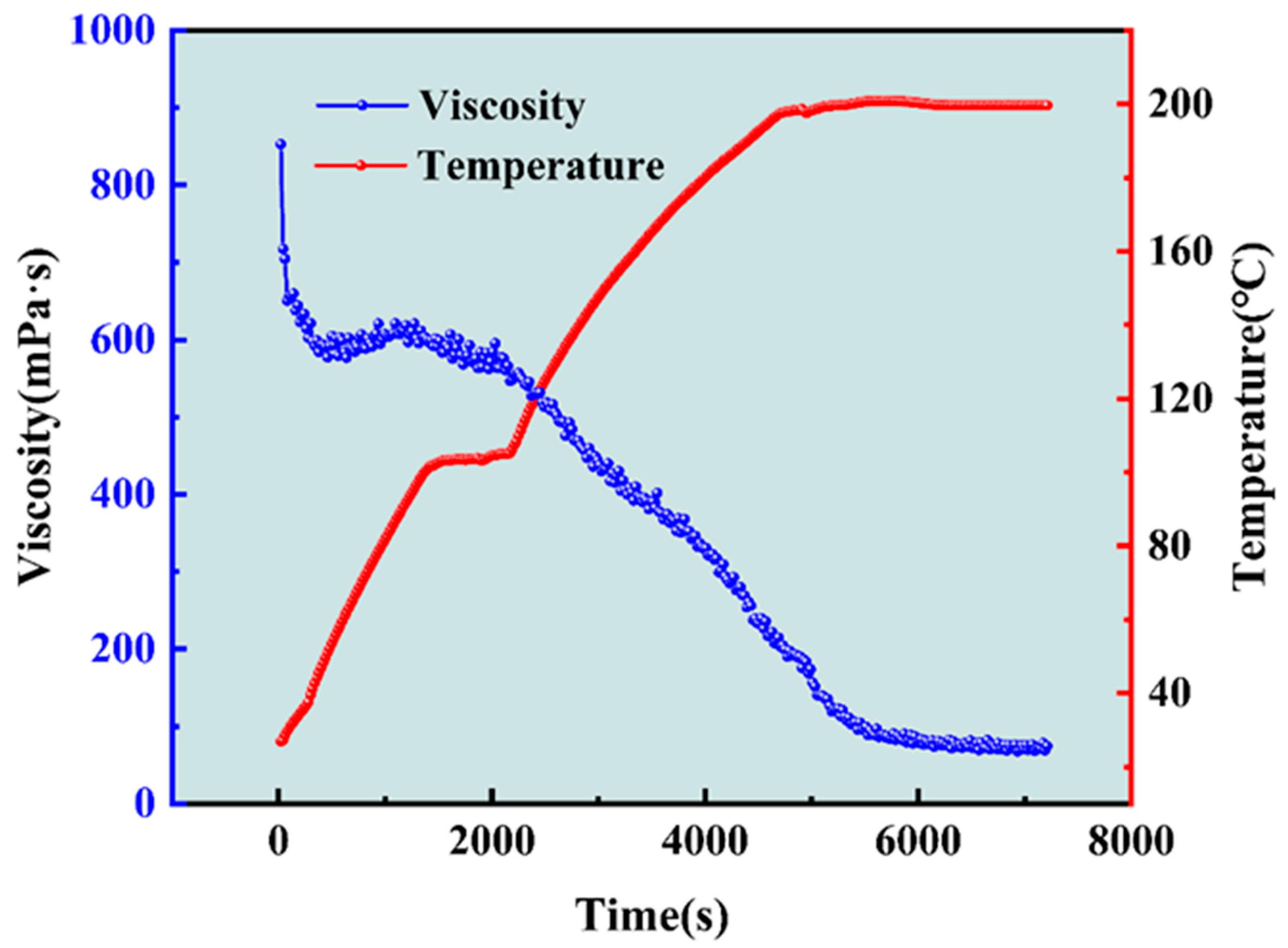
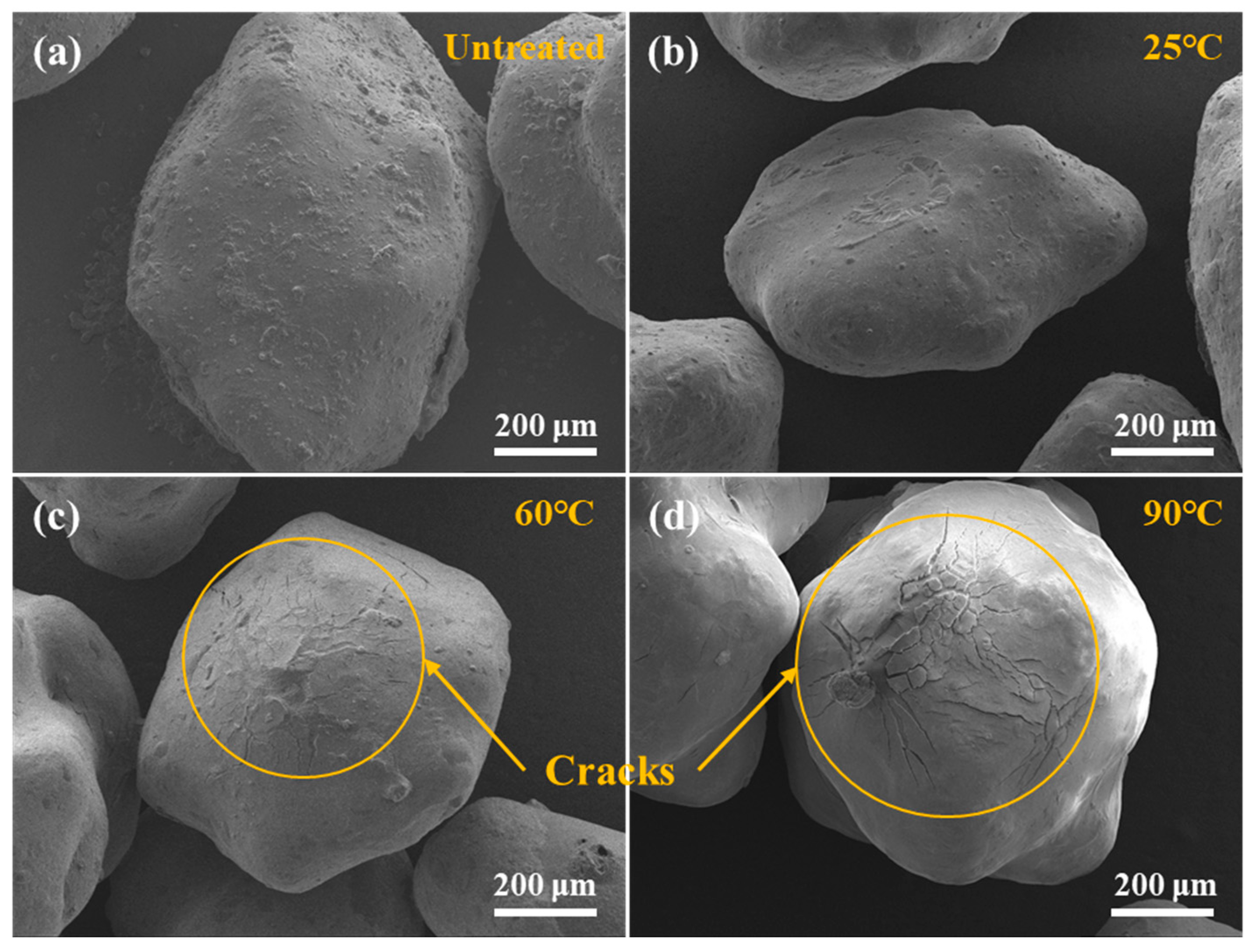
2.2. Shear Resistance of Gel Fracturing Fluid at High Temperatures
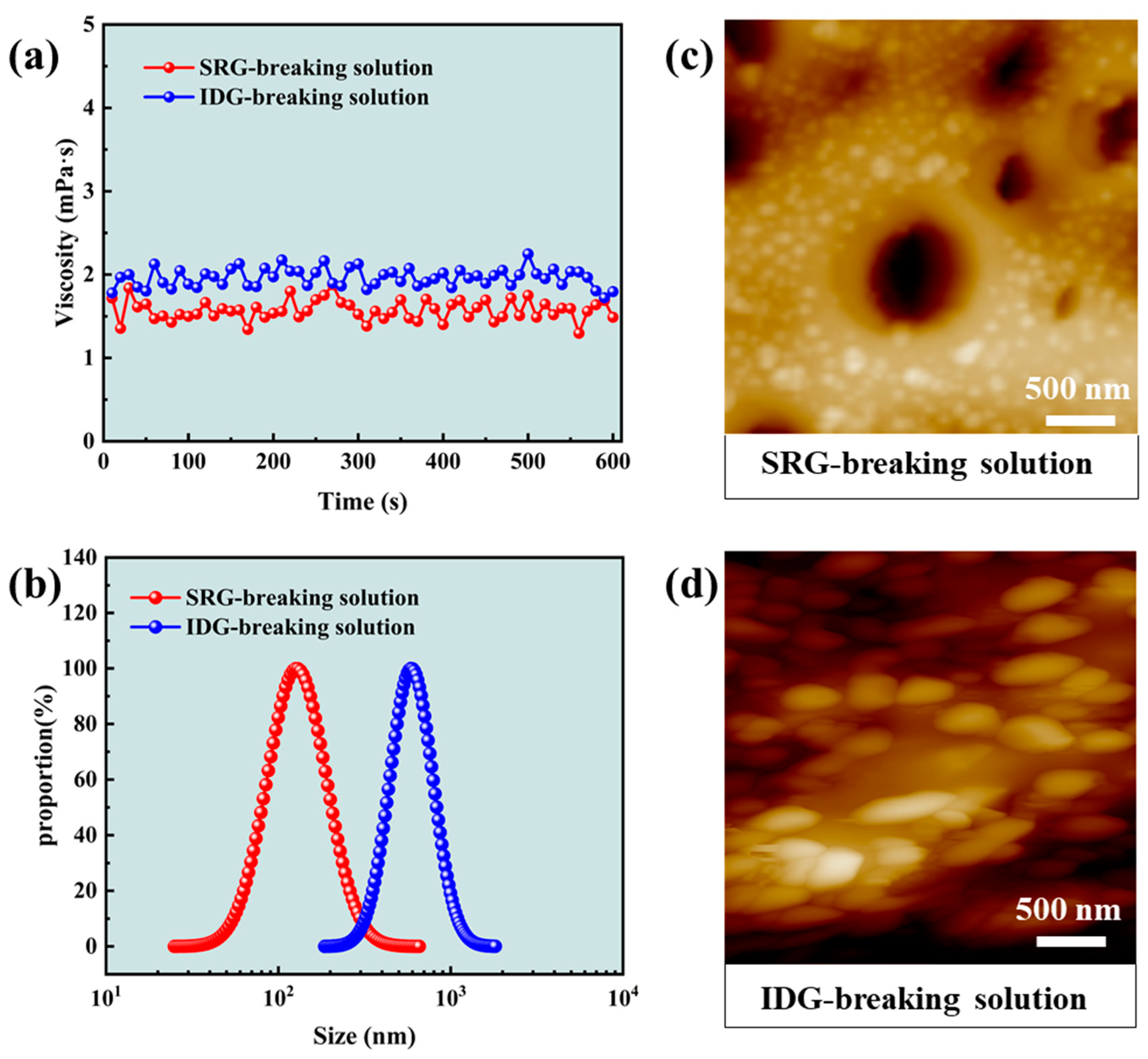
2.3. Gel-Breaking Properties of Gel Fracturing Fluid

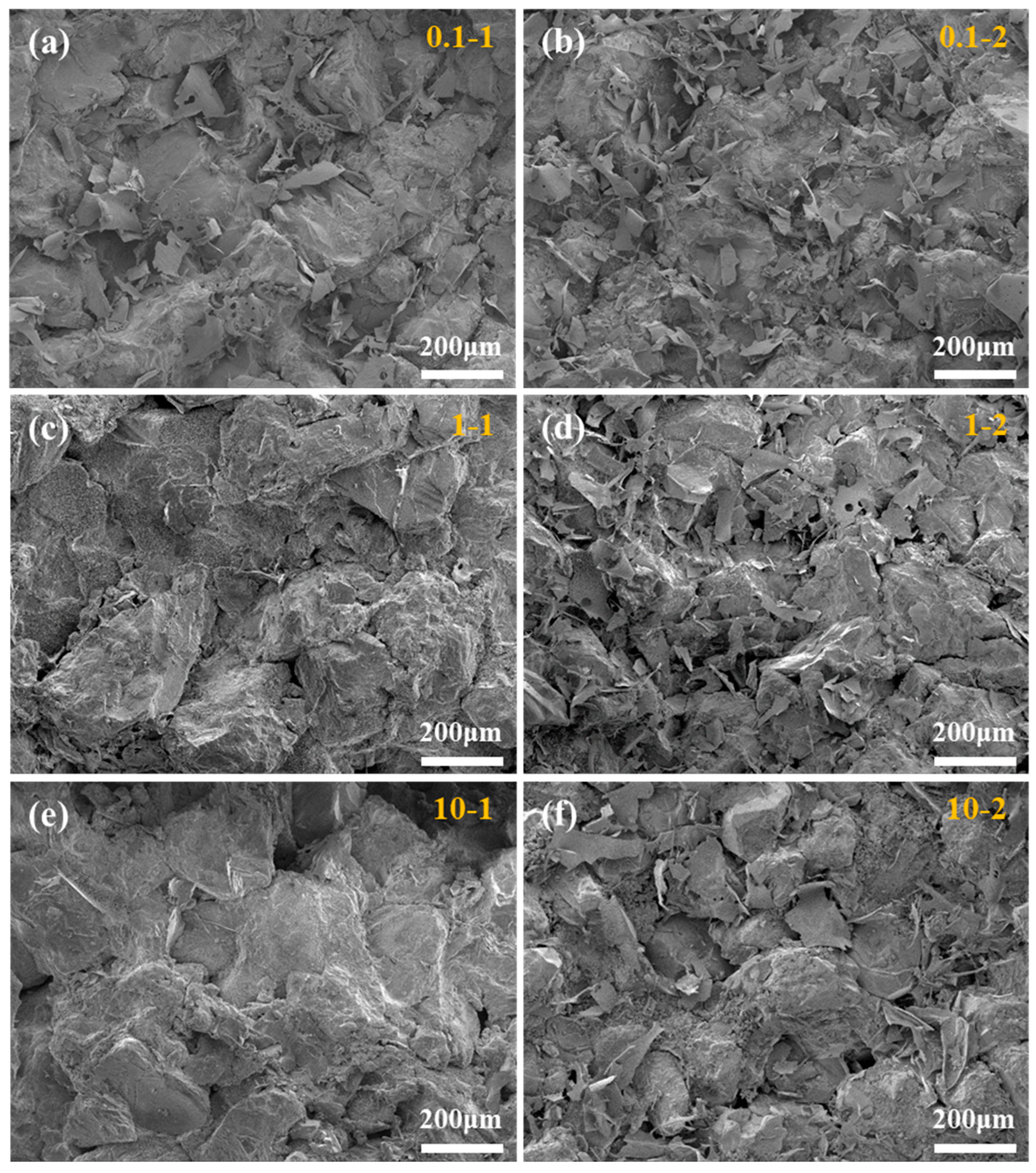
2.4. Reservoir Permeability Damage under Gel Fracturing Fluid
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of the Gel Fracturing Fluids
4.2.2. Rheological Performance Test
4.2.3. Conductivity Experiment
4.2.4. Micro-Morphology Experiment
4.2.5. Gel-Breaking Test
4.2.6. Particle Size Measurement of Gel-Breaking Solution
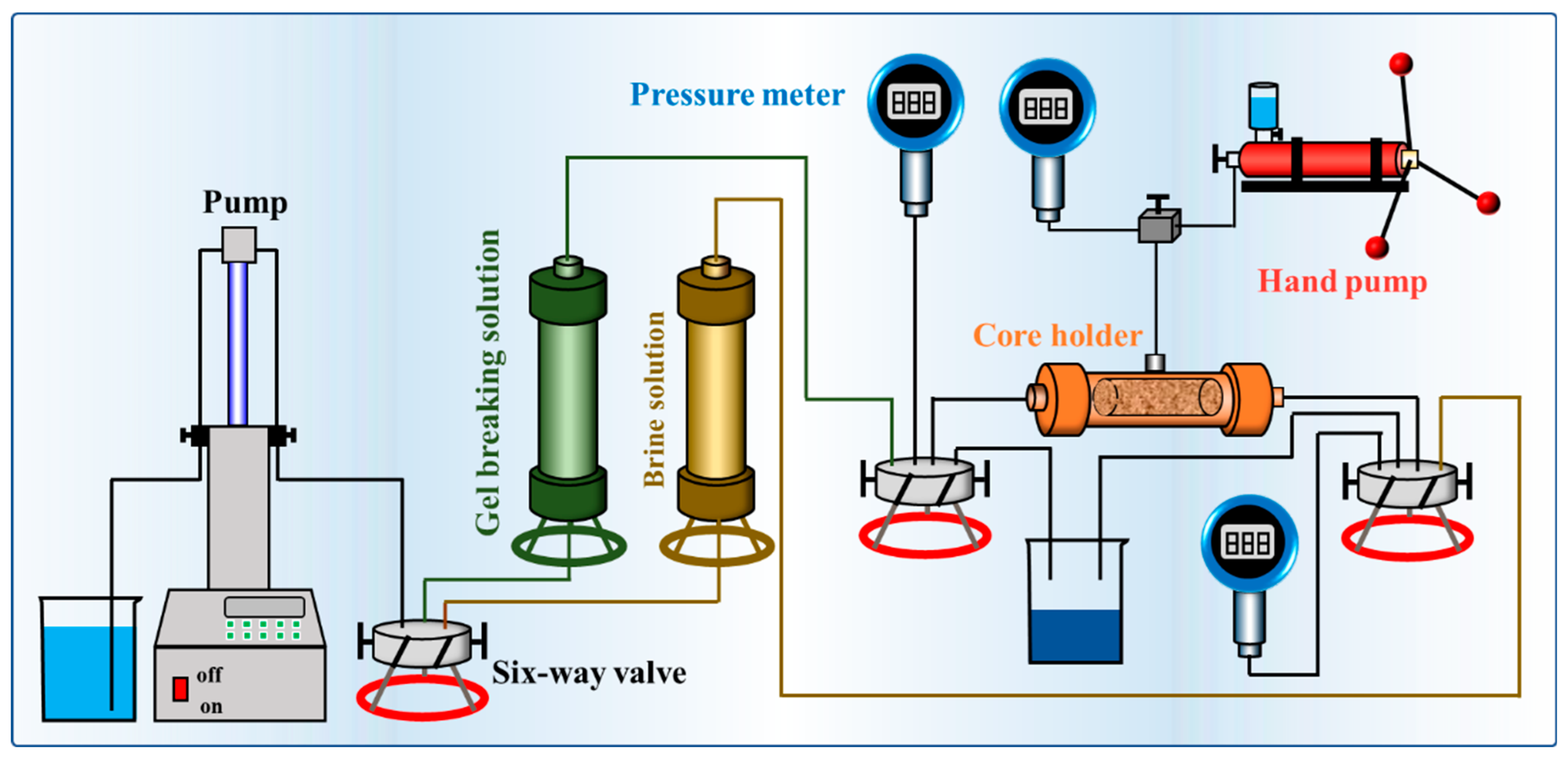
4.2.7. Permeability Damage Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Tavakkoli, O.; Kamyab, H.; Shariati, M.; Mohamed, A.M.; Junin, R. Effect of nanoparticles on the performance of polymer/surfactant flooding for enhanced oil recovery: A review. Fuel 2022, 312, 122867. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.; He, Z.; Hu, Y.; Zhao, X.; Sun, L.; Ren, H.; Dai, C. Cation-Dependent Oil-Rock Interactions in Nanopores: Insights from Core Flooding Experiments and Molecular Dynamics Simulations. Energy Fuels 2023, 37, 16564–16572. [Google Scholar]
- Malozyomov, B.V.; Martyushev, N.V.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Wu, X.; Tyncheko, Y.A.; Kukartsev, V.A. Overview of Methods for Enhanced Oil Recovery from Conventional and Unconventional Reservoirs. Energies 2023, 16, 4907. [Google Scholar] [CrossRef]
- Hao, F. Enrichment Mechanism and Prospects of Deep Oil and Gas. Acta Geol. Sin.-Engl. Ed. 2022, 96, 742–756. [Google Scholar] [CrossRef]
- Han, S.B.; Xiang, C.H.; Du, X.; Xie, L.F.; Bai, S.T.; Wang, C.S. Logging evaluation of deep multi-type unconventional gas reservoirs in the Songliao basin, northeast China: Implications from continental scientific drilling. Geosci. Front. 2022, 13, 101451. [Google Scholar] [CrossRef]
- Shen, W.; Zuo, L.; Ma, T.; Chen, C.; Qin, C.; Yang, L.; Xie, K. Quantitative Studies on the Characterization and Evaluation of Adsorbed Gas and Free Gas in Deep Shale Reservoirs. Energy Fuels 2023, 37, 3752–3759. [Google Scholar] [CrossRef]
- Cao, P.; Liu, J.S.; Leong, Y.K. General Gas Permeability Model for Porous Media: Bridging the Gaps Between Conventional and Unconventional Natural Gas Reservoirs. Energy Fuels 2016, 30, 5492–5505. [Google Scholar] [CrossRef]
- Ali, A.; Potter, D.K. Thermomagnetic Analyses of the Permeability-Controlling Minerals in Red and White Sandstones in Deep Tight Gas Reservoirs: Implications for Downhole Measurements. Spe Reserv. Eval. Eng. 2011, 14, 557–565. [Google Scholar] [CrossRef]
- Khormali, A. Effect of water cut on the performance of an asphaltene inhibitor package: Experimental and modeling analysis. Pet. Sci. Technol. 2022, 40, 2890–2906. [Google Scholar] [CrossRef]
- Lei, Q.; Xu, Y.; Yang, Z.; Cai, B.; Wang, X.; Zhou, L.; Liu, H.; Xu, M.; Wang, L.; Li, S. Progress and development directions of stimulation techniques for ultra-deep oil and gas reservoirs. Pet. Explor. Dev. 2021, 48, 221–231. [Google Scholar] [CrossRef]
- Wang, F.J.; Xu, H.; Liu, Y.K.; Meng, X.H.; Liu, L.C.F. Mechanism of low chemical agent adsorption by high pressure for hydraulic fracturing-assisted oil displacement technology: A study of molecular dynamics combined with laboratory experiments. Langmuir 2023, 39, 16628–16636. [Google Scholar] [CrossRef] [PubMed]
- He, J.M.; Lin, C.; Li, X.; Zhang, Y.X.; Chen, Y. Initiation, propagation, closure and morphology of hydraulic fractures in sandstone cores. Fuel 2017, 208, 65–70. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zou, Y.; Zhang, Y.; Wang, L.Y.; Liu, D.Q.; Sun, J.; Ge, H.K.; Zhou, D.S. Experimental study on characteristics and mechanisms of matrix pressure transmission near the fracture surface during post-fracturing shut-in in tight oil reservoirs. J. Pet. Sci. Eng. 2022, 219, 111133. [Google Scholar] [CrossRef]
- Wu, Y.T.; Pan, Z.J.; Zhang, D.Y.; Down, D.I.; Lu, Z.H.; Connell, L.D. Experimental study of permeability behaviour for proppant supported coal fracture. J. Nat. Gas Sci. Eng. 2018, 51, 18–26. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A Review of Fracturing Fluid Systems Used For Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.Z.; Mao, J.C.; Tan, H.Z.; Zhang, Y.; Song, Z.F. Review of friction reducers used in slickwater fracturing fluids for shale gas reservoirs. J. Nat. Gas Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Lv, K.; Liu, J.; Bai, Y.; Wang, J.; Huang, X.; Jin, J.; Li, J. Comparative Studies on Thickeners as Hydraulic Fracturing Fluids: Suspension versus Powder. Gels 2022, 8, 722. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhang, J.; Dong, S.B.; Li, Y.F.; Slany, M.; Chen, G. Use of Betaine-Based Gel and Its Potential Application in Enhanced Oil Recovery. Gels 2022, 8, 351. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Zhao, Y.; Yang, H.; Li, M.; Kang, X.; Zhu, T.; Zhou, B.; Sarsenbekuly, B.; Aidarova, S. Organic Acid-Enhanced Viscoelastic Surfactant and Its Application in Fracturing Fluids. Energy Fuels 2021, 35, 3130–3139. [Google Scholar] [CrossRef]
- Liu, P.; Dai, C.; Gao, M.; Wang, X.; Liu, S.; Jin, X.; Li, T.; Zhao, M. Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C. Gels 2022, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.M.; Liu, S.M.; Wang, G.; Wu, B.; Zhang, Y.Z. Coalbed methane reservoir stimulation using guar-based fracturing fluid: A review. J. Nat. Gas Sci. Eng. 2019, 66, 107–125. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Zhang, G.D.; Li, Z.J.; Hou, J.R. Laboratory Evaluation of a Low pH and Low Polymer Concentration Zirconium-CMHPG Gel System for Hydraulic Fracturing. Energy Fuels 2019, 33, 9720–9735. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, Y.L.; Xu, N.; Gong, J.C.; Liang, S.A. Synthesis and Crosslinking Mechanism of Colloidal Graphene Oxide Crosslinker for Crosslinking Low-Concentration Hydroxypropyl Guar Gum Fracturing Fluids. Energy Fuels 2022, 36, 14760–14770. [Google Scholar] [CrossRef]
- Xin, H.; Fang, B.; Yu, L.Y.; Lu, Y.J.; Xu, K.; Li, K.J. Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM. Gels 2023, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Hurnaus, T.; Plank, J. Behavior of Titania Nanoparticles in Cross-linking Hydroxypropyl Guar Used in Hydraulic Fracturing Fluids For Oil Recovery. Energy Fuels 2015, 29, 3601–3608. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhao, M.W.; Yang, Z.T.; Wang, X.Y.; Dai, C.L. Development and Gelation Mechanism of Ultra-High-Temperature-Resistant Polymer Gel. Gels 2023, 9, 726. [Google Scholar] [CrossRef]
- Wang, J.Y.; Holditch, S.A.; McVay, D.A. Effect of gel damage on fracture fluid cleanup and long-term recovery in tight gas reservoirs. J. Nat. Gas Sci. Eng. 2012, 9, 108–118. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Liu, P.; Li, T.; Liu, G.; Liu, Z.; Zhao, H. Investigation on the microscopic damage mechanism of fracturing fluids to low-permeability sandstone oil reservoir by nuclear magnetic resonance. J. Pet. Sci. Eng. 2022, 209, 109821. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yang, F.; Ge, Z.L.; Wang, Q.; Wang, S.Q. Influence of viscoelastic surfactant fracturing fluid on permeability of coal seams. Fuel 2017, 194, 1–6. [Google Scholar] [CrossRef]
- Donmoyer, S.; Agrawal, V.; Sharma, S.; Hakala, J.A. Effect of oxidative breakers on organic matter degradation, contaminant mobility and critical mineral release during shale-fracturing fluid interactions in the Marcellus Shale. Fuel 2023, 331, 125678. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Rossow, T.; Seiffert, S. Supramolecular Polymer Networks: Preparation, Properties, and Potential. In Supramolecular Polymer Networks and Gels; Seiffert, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–46. [Google Scholar]
- Sun, X.; Liang, X.B.; Wang, S.Z.; Lu, Y. Experimental study on the rheology of CO2 viscoelastic surfactant foam fracturing fluid. J. Pet. Sci. Eng. 2014, 119, 104–111. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Sun, X.; Gao, R.; Zeng, F.; Tontiwachwuthikul, P.; Liang, Z. Rheological properties study of foam fracturing fluid using CO2 and surfactant. Chem. Eng. Sci. 2017, 170, 720–730. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Mahmoud, M.; Kamal, M.S.; Patil, S.; Bataweel, M. Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater. Polymers 2021, 13, 2111. [Google Scholar] [CrossRef]
- Lv, K.H.; Zhang, G.D.; Bai, Y.R.; Yang, J.B. Preparation of Encapsulated Breakers for Polymer Gels and Evaluation of Their Properties. Gels 2023, 9, 387. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.C.; Wang, Q.; Feng, Y.; Shuai, Y.Y. Improving the fracturing fluid loss control for multistage fracturing by the precise gel breaking time design. J. Nat. Gas Sci. Eng. 2015, 25, 367–370. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Zuo, M.M.; Li, G.H.; Sha, J.P.; Zuo, X.B. Synthesis of ammonium persulfate microcapsule with a polyaniline shell and its controlled burst release. J. Appl. Polym. Sci. 2021, 138, 49695. [Google Scholar] [CrossRef]
- DeMella, K.C.; Raghavan, S.R. Catalyst-Loaded Capsules that Spontaneously Inflate and Violently Eject their Core. Langmuir 2019, 35, 13718–13726. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, M.; Wang, S.; Liu, X.; Gao, W.; Ma, Z.; Kong, C.; Ma, X.; Li, J. Encapsulation of potassium persulfate with ABS via coacervation for delaying the viscosity loss of fracturing fluid. J. Appl. Polym. Sci. 2019, 136, 47734. [Google Scholar] [CrossRef]
- Zou, C.; Dai, C.; Liu, Y.; You, Q.; Ding, F.; Huang, Y.; Sun, N. A novel self-degradable gel (SDG) as liquid temporary plugging agent for high-temperature reservoirs. J. Mol. Liq. 2023, 386, 122463. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhou, F.J.; Zhang, S.C.; Li, Z.; Wang, J.; Wang, Y.C. Evaluation of permeability damage caused by drilling and fracturing fluids in tight low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2019, 175, 1122–1135. [Google Scholar]
- Huang, Q.M.; Liu, S.M.; Cheng, W.M.; Wang, G. Fracture permeability damage and recovery behaviors with fracturing fluid treatment of coal: An experimental study. Fuel 2020, 282, 118809. [Google Scholar] [CrossRef]

| Gel Fracturing Fluid | Gel-Breaker | Gel-Breaking Time/min | The Viscosity of Gel-Breaking Solution/mPa·s |
|---|---|---|---|
| SRG | APS | 30–60 | 1.28 |
| SRG | Capsule-B | 90–120 | 1.57 |
| IDG | APS | 30–60 | 1.71 |
| IDG | Capsule-B | 90–120 | 1.96 |
| Core Label | Gel-Breaking Solution | Permeability K1/mD | Permeability K2/mD | Permeability Damage, η/% |
|---|---|---|---|---|
| 0.1-1 | SRG | 0.121 | 0.105 | 13.22 |
| 1-1 | SRG | 1.691 | 1.527 | 9.70 |
| 10-1 | SRG | 10.983 | 10.190 | 7.22 |
| 0.1-2 | IDG | 0.107 | 0.079 | 26.17 |
| 1-2 | IDG | 1.834 | 1.523 | 16.96 |
| 10-0 | IDG | 9.511 | 8.461 | 11.04 |
| Gel Type | Polymer | Crosslinker | Shortened Form |
|---|---|---|---|
| Supramolecular reinforced gel | polymer A: polymer B = 2:1 | Zr | SRG |
| Single network gel | Polymer A | Zr | SNG |
| Industrial gel | Industrial polymer | Zr | IDG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yao, X.; Dai, C.; Wu, Y.; Li, L.; Yuan, B. A Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing. Gels 2024, 10, 2. https://doi.org/10.3390/gels10010002
Huang Y, Yao X, Dai C, Wu Y, Li L, Yuan B. A Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing. Gels. 2024; 10(1):2. https://doi.org/10.3390/gels10010002
Chicago/Turabian StyleHuang, Yongping, Xinlong Yao, Caili Dai, Yining Wu, Lin Li, and Bin Yuan. 2024. "A Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing" Gels 10, no. 1: 2. https://doi.org/10.3390/gels10010002