Biocontrol Potential of Trichoderma asperellum Strain 576 against Exserohilum turcicum in Zea mays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Identification
2.2. Dual-Culture Antagonistic Activity Assay
2.3. Effect of Non-Volatile Substances Produced by Trichoderma Strains
2.4. Effect of Volatile Substances Produced by Trichoderma Strains
2.5. Fermentation Broth Antagonistic Assays
2.6. Enzymatic Activity
2.7. Effect of T. asperellum 576 on Maize Seed Germination
2.8. Biocontrol Effect of T. asperellum 576 against E. turcicum 101
2.9. Statistical Analysis
3. Results
3.1. Species Identification and Phylogenetic Analysis
3.2. Dual Culture Tests
3.3. Antagonistic Effect by Trichoderma Non-Volatile Substances
3.4. Fungal Growth Inhibition by Trichoderma Volatile Substances
3.5. Antifungal Activity of the Co-Culture Suspension against E. turcicum 101
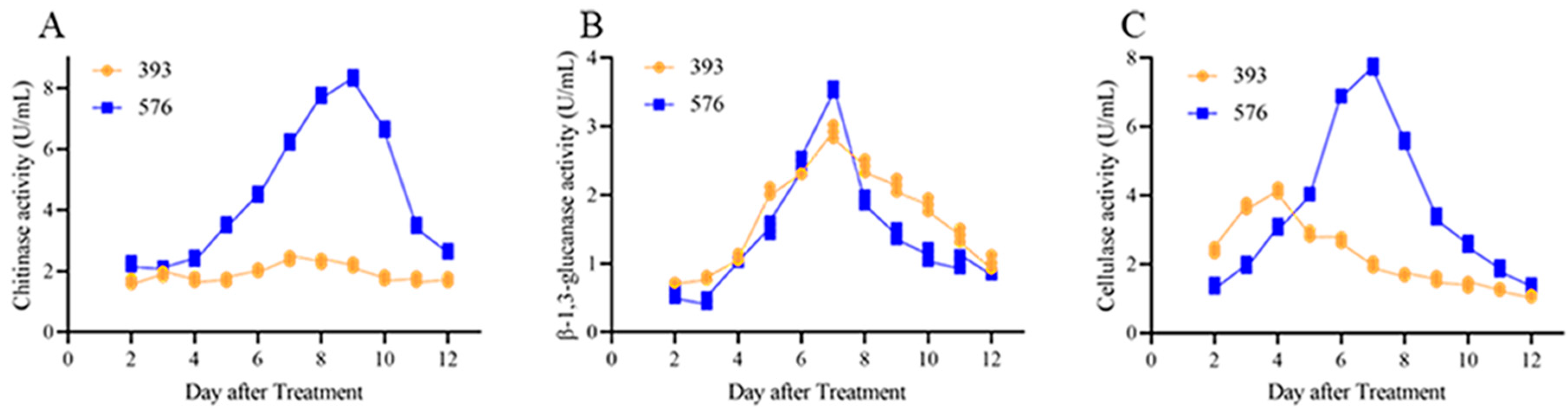
3.6. Activity of Cell Wall Degradation Enzymes
3.7. Stimulating Germination of Maize Seeds by T. asperellum 576
3.8. Effect of T. asperellum 576 on E. turcicum 101 in Maize Seedlings
4. Discussion
4.1. Evidence of Direct Mycoparasitism and Intraspecies Variability in Trichoderma-E. turcicum Interaction
4.2. Non-Volatile Metabolites of Trichoderma spp.: Potent Inhibitors of E. turcicum 101 and Antibiosis as a Key Biocontrol Mechanism
4.3. Unveiling the Pole of Trichoderma Volatile Substances in Inhibiting E. turcicum 101: A Potential Mechanism for Enhanced Biological Control
4.4. Potent Antifungal Activity of T. asperellum 576 in Co-Culture with E. turcicum 101: Implications for Endophytic Environment Establishment
4.5. Role of Cell Wall-Degrading Enzymes in the Biocontrol Potential of T. asperellum 576 against E. turcicum 101
4.6. Enhancing Maize Seed Germination with T. asperellum 576: Towards Optimal Spore Dose for Targeted Plant Growth Promotion
4.7. Exploring the Potential of T. asperellum 576 for Plant Growth Promotion and Disease Suppression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, N.; Meng, Q.; Feng, P.; Qu, Z.; Yu, Y.; Liu, D.L.; Müller, C.; Wang, P. China can be self-sufficient in maize production by 2030 with optimal crop management. Nat. Commun. 2023, 14, 2637. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bodirsky, B.L.; Dietrich, J.P.; Martinelli, E.; Stenstad, A.; Pradhan, P.; Gabrysch, S.; Mishra, A.; Weindl, I.; Le Mouël, C.; Rolinski, S.; et al. The ongoing nutrition transition thwarts long-term targets for food security, public health and environmental protection. Sci. Rep. 2020, 10, 19778. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tong, L.; Kang, S.; Du, T.; Ding, R.; Li, S.; Chen, Y. Combination of suitable planting density and nitrogen rate for high yield maize and their source-sink relationship in Northwest China. J. Sci. Food Agric. 2023, 103, 5300–5311. [Google Scholar] [CrossRef] [PubMed]
- Serna-Saldivar, S.O.; Perez Carrillo, E. Chapter 16—Food uses of whole corn and dry-milled fractions. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 435–467. [Google Scholar]
- Badu-Apraku, B.; Bankole, F.A.; Ajayo, B.S.; Fakorede, M.A.B.; Akinwale, R.O.; Talabi, A.O.; Bandyopadhyay, R.; Ortega-Beltran, A. Identification of early and extra-early maturing tropical maize inbred lines resistant to Exserohilum turcicum in sub-Saharan Africa. Crop Prot. 2021, 139, 105386. [Google Scholar] [CrossRef]
- Manu, T.G.; Naik, B.G.; Sayipratap, B.R.; Balagar, M.S. Chemical science review and letters efficacy of fungicides, botanicals and bio-agents against Exserohilum turcicum. Chem. Sci. Rev. Lett. 2017, 6, 2100–2107. [Google Scholar]
- Huang, W.; Fang, X.; Wang, H.; Chen, F.; Duan, H.; Bi, Y.; Yu, H. Biosynthesis of AgNPs by Bipolaris maydis and its antifungal effect against Exserohilum turcicum. IET Nanobiotechnol. 2018, 12, 585–590. [Google Scholar] [CrossRef]
- CABI. Crop Protection Compendium: Setosphaeria turcica (Maize Leaf Blight); CABI International CABI: Wallingford, UK, 2022; Volume CABI Compendium. [Google Scholar]
- Navarro, B.L.; Campos, R.D.; Gasparoto, M.C.D.; von Tiedemann, A. In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America. Pathogens 2021, 10, 154. [Google Scholar] [CrossRef]
- Balint-Kurti, P.J.; Yang, J.Y.; Van Esbroeck, G.; Jung, J.; Smith, M.E. Use of a maize advanced intercross line for mapping of QTL for northern leaf blight resistance and multiple disease resistance. Crop Sci. 2010, 50, 458–466. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Wiebold, W.J. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 2020, 21, 238–247. [Google Scholar] [CrossRef]
- Weems, J.D.; Bradley, C.A. Sensitivity of Exserohilum turcicum to demethylation inhibitor fungicides. Crop Prot. 2017, 99, 85–92. [Google Scholar] [CrossRef]
- Mahrous, N.N.; Columbus, M.P.; Southam, G.; Macfie, S.M. Changes in microbial community structure and increased metal bioavailability in a metal-contaminated soil and in the rhizosphere of corn (Zea mays). Rhizosphere 2019, 11, 100169. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Chapter 5—Actinobacteria: Eco-friendly candidates for control of plant diseases in a sustainable manner. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 79–91. [Google Scholar]
- Fazeli-Nasab, B.; Shahraki-Mojahed, L.; Piri, R.; Sobhanizadeh, A. 20—Trichoderma: Improving growth and tolerance to biotic and abiotic stresses in plants. In Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.C., Yadav, A.N., Goel, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 525–564. [Google Scholar]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G.-d. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Nesci, A.; Formento, Á.; Etcheverry, M. Selection of potential biological control of Exserohilum turcicum with epiphytic microorganisms from maize. Rev. Argent. Microbiol. 2015, 47, 62–71. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Muhie, S.H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Ahmad, F.; Saeed, Q.; Shah, S.M.U.; Gondal, M.A.; Mumtaz, S. Chapter 11—Environmental sustainability: Challenges and approaches. In Natural Resources Conservation and Advances for Sustainability; Jhariya, M.K., Meena, R.S., Banerjee, A., Meena, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–270. [Google Scholar]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Samuels, G.J. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 2006, 96, 195–206. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Lin, X.; Tang, Z.; Gan, Y.; Li, Z.; Luo, X.; Gao, C.; Zhao, L.; Chai, L.; Liu, Y. 18-Residue peptaibols produced by the sponge-derived Trichoderma sp. GXIMD 01001. J. Nat. Prod. 2023, 86, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Tang, W.L.; Huang, Q.R.; Li, Y.Z.; Wei, M.L.; Jiang, L.L.; Liu, C.; Yu, X.; Zhu, H.W.; Chen, G.Z.; et al. Trichoderma: A treasure house of structurally diverse secondary metabolites with medicinal importance. Front. Microbiol. 2021, 12, 723828. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 25-IN3. [Google Scholar] [CrossRef]
- Li, M.-F.; Li, G.-H.; Zhang, K.-Q. Non-Volatile Metabolites from Trichoderma spp. Metabolites 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ghirardo, A.; Weber, B.; Schnitzler, J.-P.; Benz, J.P.; Rosenkranz, M. Trichoderma Species Differ in Their Volatile Profiles and in Antagonism Toward Ectomycorrhiza Laccaria bicolor. Front. Microbiol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef]
- Lee, S.; Behringer, G.; Hung, R.; Bennett, J. Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol. 2019, 37, 1–9. [Google Scholar] [CrossRef]
- Siddiquee, S. Chapter 11—Recent Advancements on the Role and Analysis of Volatile Compounds (VOCs) from Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 139–175. [Google Scholar]
- Limdolthamand, S.; Songkumarn, P.; Suwannarat, S.; Jantasorn, A.; Dethoup, T. Biocontrol efficacy of endophytic Trichoderma spp. in fresh and dry powder formulations in controlling northern corn leaf blight in sweet corn. Biol. Control 2023, 181, 105217. [Google Scholar] [CrossRef]
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum confer growth promotion and protection against late wilt disease in the field. J. Fungi 2021, 7, 444. [Google Scholar] [CrossRef]
- Shang, J.; Liu, B.; Xu, Z. Efficacy of Trichoderma asperellum TC01 against anthracnose and growth promotion of Camellia sinensis seedlings. Biol. Control 2020, 143, 104205. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Samuels, G.J.; Ismaiel, A.; Voglmayr, H. Disentangling the Trichoderma viridescens complex. Persoonia 2013, 31, 112–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.X.; Zhuang, W.Y. Trichoderma (Hypocrea) species with green ascospores from China. Persoonia 2015, 34, 113–129. [Google Scholar] [CrossRef]
- Chaverri, P.; Samuels, G.J. Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores. Stud. Mycol. 2003, 48, 1–116. [Google Scholar]
- Jaklitsch, W.M.; Komon, M.; Kubicek, C.P.; Druzhinina, I.S. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 2005, 97, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M. European species of Hypocrea Part I. The green-spored species. Stud. Mycol. 2009, 63, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhuang, W.Y. First step evaluation of Trichoderma antagonism against plant pathogenic fungi in dual culture. Mycosystema 2017, 36, 1251–1259. [Google Scholar]
- Joo, J.H.; Hussein, K.A. Biological control and plant growth promotion properties of volatile organic compound-producing antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef] [PubMed]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature differentially influences the capacity of Trichoderma species to induce plant defense responses in tomato against insect pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Jiang, L.; Meng, Q.; Zhang, G.; Song, Z. Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J. Basic Microbiol. 2012, 52, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 420–428. [Google Scholar] [CrossRef]
- Singh, D.B.; Gupta, R.K.; Singh, R.; Patil, R. Minimal processing of papaya for quality maintenance and shelf life. Acta Hortic. 2010, 851, 579–590. [Google Scholar] [CrossRef]
- EI-gamal, d.n.; Atalla, S.; El-Mohamedy, R.R.S. Improvement in potential of enzymes from Chaetomium globosum and Trichoderma harzianum using different agricultural wastes and its applications. Biosci. Res. 2018, 15, 3977–3987. [Google Scholar]
- Almeida, F.B.; Cerqueira, F.M.; Silva Rdo, N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: Evaluation of coiling and hydrolytic enzyme production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Ran, W.; Shen, Q.; Yang, X. Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl. Microbiol. Biotechnol. 2011, 91, 741–755. [Google Scholar] [CrossRef]
- Monteiro, V.N.; do Nascimento Silva, R.; Steindorff, A.S.; Costa, F.T.; Noronha, E.F.; Ricart, C.A.; de Sousa, M.V.; Vainstein, M.H.; Ulhoa, C.J. New insights in Trichoderma harzianum antagonism of fungal plant pathogens by secreted protein analysis. Curr. Microbiol. 2010, 61, 298–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, W.-Y. Trichoderma brevicrassum strain TC967 with capacities of diminishing cucumber disease caused by Rhizoctonia solani and promoting plant growth. Biol. Control 2020, 142, 104151. [Google Scholar] [CrossRef]
- Pradhan, D.A.; Bagagoni, P.; Makandar, R. Assessing rhizosphere Trichoderma asperellum strains for root colonizing and antagonistic competencies against Fusarium wilt through molecular and biochemical responses in castor. Biol. Control 2023, 184, 105280. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef] [PubMed]
- Mbarga, J.B.; Begoude, B.A.D.; Ambang, Z.; Meboma, M.; Kuate, J.; Ewbank, W.; Hoopen, G.M.t. Field testing an oil-based Trichoderma asperellum formulation for the biological control of cacao black pod disease, caused by Phytophthora megakarya. Crop Prot. 2020, 132, 105134. [Google Scholar] [CrossRef]
- Shanmugam, V.; Varma, A.S. Effect of native antagonists against Pythium aphanidermatum, the casual organism of rhizome rot of ginger. J. Mycol. Pl. Pathol. 1999, 29, 375–379. [Google Scholar]
- Kucuk, C.; Kivanc, M. In vitro antifungal activity of strains of Trichoderma harzianum. Turk. J. Biol. 2004, 28, 111–115. [Google Scholar]
- Lee, J.; Huh, N.; Hong, J.H.; Kim, B.S.; Kim, G.-H.; Kim, J.-J. The antagonistic properties of Trichoderma spp. inhabiting woods for potential biological control of wood-damaging fungi. Holzforschung 2012, 66, 883–887. [Google Scholar] [CrossRef]
- Amin, F.; Razdan, V.; Mohiddin, F.; Bhat, K.; Sheikh, P. Effect of volatile metabolites of Trichoderma species against seven fungal plant pathogens In-Vitro. J. Phytol. 2010, 2, 34–37. [Google Scholar]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Woo, S.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Murphy, B.; Batke, S.; Doohan, F.; Hodkinson, T. Media manipulations and the culture of beneficial fungal root endophytes. Int. J. Biol. 2015, 7, 94–102. [Google Scholar] [CrossRef]
- Carsolio, C.; Benhamou, N.; Haran, S.; Cortés, C.; Gutiérrez, A.; Chet, I.; Herrera-Estrella, A. Role of the Trichoderma harzianum endochitinase gene, ech42, in mycoparasitism. Appl. Environ. Microbiol. 1999, 65, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Ano, A.; Takayanagi, T.; Uchibori, T.; Okuda, T.; Yokotsuka, K. Characterization of a class III chitinase from Vitis vinifera cv. Koshu. J. Biosci. Bioeng. 2003, 95, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Bech, L.; Busk, P.; Lange, L. Cell wall degrading enzymes in Trichoderma asperellum grown on wheat bran. Fungal Genom. Biol. 2014, 4, 1000116. [Google Scholar]
- Moussa, Z.; Alanazi, Y.F.; Khateb, A.M.; Eldadamony, N.M.; Ismail, M.M.; Saber, W.I.A.; Darwish, D.B.E. Domiciliation of Trichoderma asperellum suppresses Globiosporangium ultimum and promotes pea growth, ultrastructure, and metabolic features. Microorganisms 2023, 11, 198. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Shabbir, M.Z.; Wang, Y.; Ahmed, M.A.; Guo, J.; He, K.; Zhang, T.; Bai, S.; Chen, J.; et al. Seed Myco-priming improves crop yield and herbivory induced defenses in maize by coordinating antioxidants and Jasmonic acid pathway. BMC Plant Biol. 2022, 22, 554. [Google Scholar] [CrossRef]
- Singh, V.; Upadhyay, R.S.; Sarma, B.K.; Singh, H.B. Trichoderma asperellum spore dose depended modulation of plant growth in vegetable crops. Microbiol. Res. 2016, 193, 74–86. [Google Scholar] [CrossRef]
- Björkman, T.; Blanchard, L.; Harman, G. Growth enhancement of shrunken-2 (sh2) sweet corn by Trichoderma harzianum 1295-22: Effect of environmental stress. J. Am. Soc. Hortic. Sci. 1998, 123, 35–40. [Google Scholar] [CrossRef]
- Batta, Y.A. Effect of treatment with Trichoderma harzianum Rifai formulated in invert emulsion on postharvest decay of apple blue mold. Int. J. Food Microbiol. 2004, 96, 281–288. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, A.; Chaudhary, A.; Singh, S.; Singh, H.B. Modulation of nutritional and antioxidant potential of seeds and pericarp of pea pods treated with microbial consortium. Food Res. Int. 2014, 64, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.S.; Druzhinina, I.; Tuohy, M. Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–549. [Google Scholar]
- Singh, A.; Sarma, B.K.; Singh, H.B.; Upadhyay, R.S. Chapter 40—Trichoderma: A silent worker of plant Rhizosphere. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 533–542. [Google Scholar]
- Abdelkhalek, A.; Hafez, E.; Aboelhana, E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with Tobacco mosaic virus. Biosci. Res. 2019, 4, 3349–3356. [Google Scholar]
- Rubio, M.B.; Monti, M.M.; Gualtieri, L.; Ruocco, M.; Hermosa, R.; Monte, E. Trichoderma harzianum volatile organic compounds regulated by the THCTF1 transcription factor are involved in antifungal activity and beneficial plant responses. J. Fungi 2023, 9, 654. [Google Scholar] [CrossRef]
- Karuppiah, V.; Zhang, C.; Liu, T.; Li, Y.; Chen, J. Transcriptome Analysis of T. asperellum GDFS 1009 Revealed the Role of MUP1 Gene on the Methionine-Based Induction of Morphogenesis and Biological Control Activity. J. Fungi 2023, 9, 215. [Google Scholar] [CrossRef]
- Saharan, R.; Patil, J.A.; Yadav, S.; Kumar, A.; Goyal, V. The nematicidal potential of novel fungus, Trichoderma asperellum FbMi6 against Meloidogyne incognita. Sci. Rep. 2023, 13, 6603. [Google Scholar] [CrossRef]
- Zapata-Sarmiento, D.H.; Palacios-Pala, E.F.; Rodríguez-Hernández, A.A.; Medina Melchor, D.L.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Trichoderma asperellum, a potential biological control agent of Stemphylium vesicarium, on onion (Allium cepa L.). Biol. Control 2020, 140, 104105. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, R.; Ni, M.; Yu, J.; Li, Y.; Yu, C.; Dou, K.; Ren, J.; Chen, J. Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef]
- Ou, T.; Zhang, M.; Gao, H.; Wang, F.; Xu, W.; Liu, X.; Wang, L.; Wang, R.; Xie, J. Study on the potential for stimulating mulberry growth and drought tolerance of plant growth-promoting fungi. Int. J. Mol. Sci. 2023, 24, 4090. [Google Scholar] [CrossRef]
- Irshad, K.; Shaheed Siddiqui, Z.; Chen, J.; Rao, Y.; Hamna Ansari, H.; Wajid, D.; Nida, K.; Wei, X. Bio-priming with salt tolerant endophytes improved crop tolerance to salt stress via modulating photosystem II and antioxidant activities in a sub-optimal environment. Front. Plant Sci. 2023, 14, 1082480. [Google Scholar] [CrossRef]







| Taxon | Strain/Specimen | GenBank Accession Number | |
|---|---|---|---|
| TEF1-α | RPB2 | ||
| Trichoderma atroviride | 393 | OR548045 1 | OR548096 |
| T. afroharzianum | XZ9-1 | OR548046 | OR548111 |
| T. breve | 578 | OR548049 | - |
| T. brevicompactum | 592 | OR548083 | OR548105 |
| T. cerinum | XZ1-3 | OR548077 | OR548110 |
| T. chromospermum | 91 | OR548054 | - |
| T. chromospermum | 338 | OR548066 | - |
| T. crassum | 110 | KT149299 | - |
| T. guizhouense | 526 | OR548073 | - |
| T. guizhouense | TU2 | OR548076 | OR548109 |
| T. hamatum | MSL-3 | OR548084 | - |
| T. harzianum | 581 | OR548050 | OR548103 |
| T. koningiopsis | 421 | OR548081 | OR548099 |
| T. koningiopsis | 439 | OR548071 | OR548101 |
| T. linzhiense | 449 | OR548072 | - |
| T. longibrachiatum | 539 | OR548074 | OR548102 |
| T. longibrachiatum | 3a | OR548053 | - |
| T. longifialidicum | 224 | OR548063 | OR548091 |
| T. longipile | L-3 | OR548075 | - |
| T. dorothopsis | 438 | OR548087 | OR548100 |
| T. pararogersonii | 219 | OR548062 | - |
| T. paratroviride | 402 | OR548069 | OR548097 |
| T. paraviridescens | 295 | OR548079 | OR548092 |
| T. petersenii | 509 | OR548047 | - |
| T. pleuroticola | 588 | OR548082 | OR548104 |
| T. polysporum | 1408 | OR548052 | - |
| T. pyramidale | 285 | OR548064 | - |
| T. rodmanii | 299 | OR548065 | - |
| T. rodmanii | 376 | OR548080 | OR548095 |
| T. endophyticum | 99 | KX689257 | OR548108 |
| T. auriculariae | 417 | OR548070 | OR548098 |
| T. sinense | 204 | OR548060 | - |
| T. solum | 375 | OR548068 | OR548094 |
| T. stipitatum | 218 | OR548061 | - |
| T. strictipile | 370 | OR548078 | - |
| T. strictipile | 115 | OR548058 | OR548089 |
| T. thailandicum | 1283 | OR548086 | OR548090 |
| T. thelephoricola | 342 | OR548067 | OR548093 |
| T. tomentosum | 153 | OR548059 | - |
| T. vinosum | XZ5-2 | OR548085 | - |
| T. longibrachiatum | 593 | OR548051 | OR548106 |
| T. zonatum | 220 | MF374809 | MF374806 |
| T. italicum | 64 | OR548054 | OR548107 |
| T. asperellum | 576 | OR548047 | OR548088 |
| T. asperellum | HZA10 | MK850832 | MH647800 |
| T. atroviride | CBS 119499 | FJ860611 | FJ860518 |
| T. breve | HMAS248845 | KY688046 | KY687984 |
| T. compactum | CBS 121218 | KF134798 | KP115276 |
| T. evansil | Dis 282d | EU856319 | FJ150784 |
| T. helicolixii | CBS 133499 | KJ665517 | KJ665278 |
| T. italicum | CBS 132567 | KJ665525 | KJ665282 |
| T. harzianum | CBS 226.95 | AF534621 | AF545549 |
| T. guizhouense | S628 | KJ665511 | KJ665273 |
| T. hamatum | Th23 | OL439486 | OL412667 |
| T. longibrachiatum | CBS 816.68 | AY865640 | DQ087242 |
| T. polysporum | CPK 3131 | FJ860661 | JQ685878 |
| T. rodmanii | CBS 121553 | FJ860687 | FJ860580 |
| T. koningiopsis | GJS 93-20 | DQ284966 | EU241506 |
| T. paratroviride | CBS136489 | KJ665627 | KJ665321 |
| T. brevicompactum | CBS 109720 | OP203936 | OP203935 |
| T. pseudolacteum | TUFC 61490 | JX238493 | JX238478 |
| Strains | Colony Diameter (mm) | Inhibition Rate (%) |
|---|---|---|
| 576 | 11.18 | 80.81 ± 2.01 a 1 |
| 393 | 13.29 | 77.68 ± 3.87 ab |
| 421 | 15.84 | 73.41 ± 2.70 ab |
| 110 | 18.35 | 69.20 ± 1.23 ab |
| 3A | 20.35 | 65.84 ± 5.60 bc |
| XZ9-1 | 27.64 | 53.60 ± 4.36 cd |
| 285 | 27.95 | 53.07 ± 2.94 d |
| 539 | 27.95 | 53.07 ± 2.94 d |
| 417 | 34.42 | 42.22 ± 2.02 de |
| XZ1-3 | 37.25 | 37.46 ± 2.44 ef |
| 342 | 40.21 | 32.49 ± 3.01 ef |
| TU2 | 40.64 | 31.77 ± 5.10 ef |
| 64 | 43.92 | 26.27 ± 3.30 fg |
| 402 | 44.82 | 24.75 ± 6.14 g |
| CK | 59.56 | - |
| Strain | Colony Diameter (mm) | Inhibition Rate (%) |
|---|---|---|
| 576 | 14.72 | 65.86 ± 0.27 a 1 |
| 393 | 18.31 | 57.54 ± 0.71 ab |
| 421 | 19.9 | 53.85 ± 0.34 b |
| 110 | 29.12 | 32.47 ± 0.75 c |
| 3A | 33.16 | 23.10 ± 0.83 d |
| CK | 43.12 | - |
| Day | T. asperellum 576 + E. turcicum 101 | T. atroviride 393 + E. turcicum 101 | ||
|---|---|---|---|---|
| Colony Diameter (mm) | Inhibition Rate (%) | Colony Diameter (mm) | Inhibition Rate (%) | |
| 2 | 48.79 | 12.48 ± 2.69 gh 1 | 41.53 | 25.50 ± 3.53 h |
| 3 | 40.18 | 27.93 ± 4.32 e | 36.86 | 34.12 ± 2.13 fg |
| 4 | 31.07 | 44.26 ± 3.42 cd | 31.90 | 43.02 ± 1.95 e |
| 5 | 28.19 | 50.43 ± 3.63 bc | 30.20 | 46.22 ± 1.41 de |
| 6 | 25.85 | 53.63 ± 0.64 b | 20.94 | 62.50 ± 4.95 a |
| 7 | 19.82 | 64.45 ± 2.52 a | 27.69 | 50.50 ± 0.71 cd |
| 8 | 34.04 | 54.94 ± 3.15 b | 24.08 | 57.00 ± 1.39 b |
| 9 | 44.09 | 50.91 ± 4.23 bc | 25.75 | 54.03 ± 1.88 bc |
| 10 | 46.41 | 39.74 ± 0.84 de | 28.40 | 49.25 ± 2.37 cd |
| 11 | 47.20 | 15.32 ± 0.86 fg | 35.29 | 36.50 ± 2.12 f |
| 12 | 51.84 | 10.71 ± 3.78 h | 38.27 | 31.14 ± 2.83 g |
| CK | 55.74 | - | - | - |
| Treatment | Shoot Height (cm) | Stem Diameter (cm) | Fresh Shoot (g) | Fresh Root (g) | Dry Shoot (g) | Dry Root (g) |
|---|---|---|---|---|---|---|
| T1 | 70.00 ± 2.00 b 1 | 0.55 ± 0.03 b | 9.42 ± 1.18 b | 3.44 ± 0.40 b | 2.00 ± 0.15 b | 1.65 ± 0.18 b |
| T2 | 82.00 ± 1.73 a | 0.65 ± 0.00 a | 14.37 ± 1.86 a | 4.67 ± 0.55 a | 2.97 ± 0.26 a | 2.43 ± 0.06 a |
| T3 | 60.33 ± 1.53 c | 0.44 ± 0.05 c | 5.17 ± 1.26 c | 1.65 ± 0.67 c | 1.03 ± 0.49 c | 0.73 ± 0.40 c |
| T4 | 65.67 ± 3.21 b | 0.51± 0.20 b | 7.50 ± 0.50 c | 2.79 ± 0.24 b | 1.77 ± 0.17 b | 1.42 ± 0.19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, Y.; Yang, S.; Li, Y.; Zhu, Z. Biocontrol Potential of Trichoderma asperellum Strain 576 against Exserohilum turcicum in Zea mays. J. Fungi 2023, 9, 936. https://doi.org/10.3390/jof9090936
Ma Y, Li Y, Yang S, Li Y, Zhu Z. Biocontrol Potential of Trichoderma asperellum Strain 576 against Exserohilum turcicum in Zea mays. Journal of Fungi. 2023; 9(9):936. https://doi.org/10.3390/jof9090936
Chicago/Turabian StyleMa, Yukun, Yetong Li, Shijia Yang, Yu Li, and Zhaoxiang Zhu. 2023. "Biocontrol Potential of Trichoderma asperellum Strain 576 against Exserohilum turcicum in Zea mays" Journal of Fungi 9, no. 9: 936. https://doi.org/10.3390/jof9090936






