Evaluation of the Mycorrhizal Potential of Date Palm (Phoenix dactylifera L.) Rhizosphere Soils in the Figuig Oasis (Southeastern Morocco)
Abstract
:1. Introduction
2. Materials and Methods
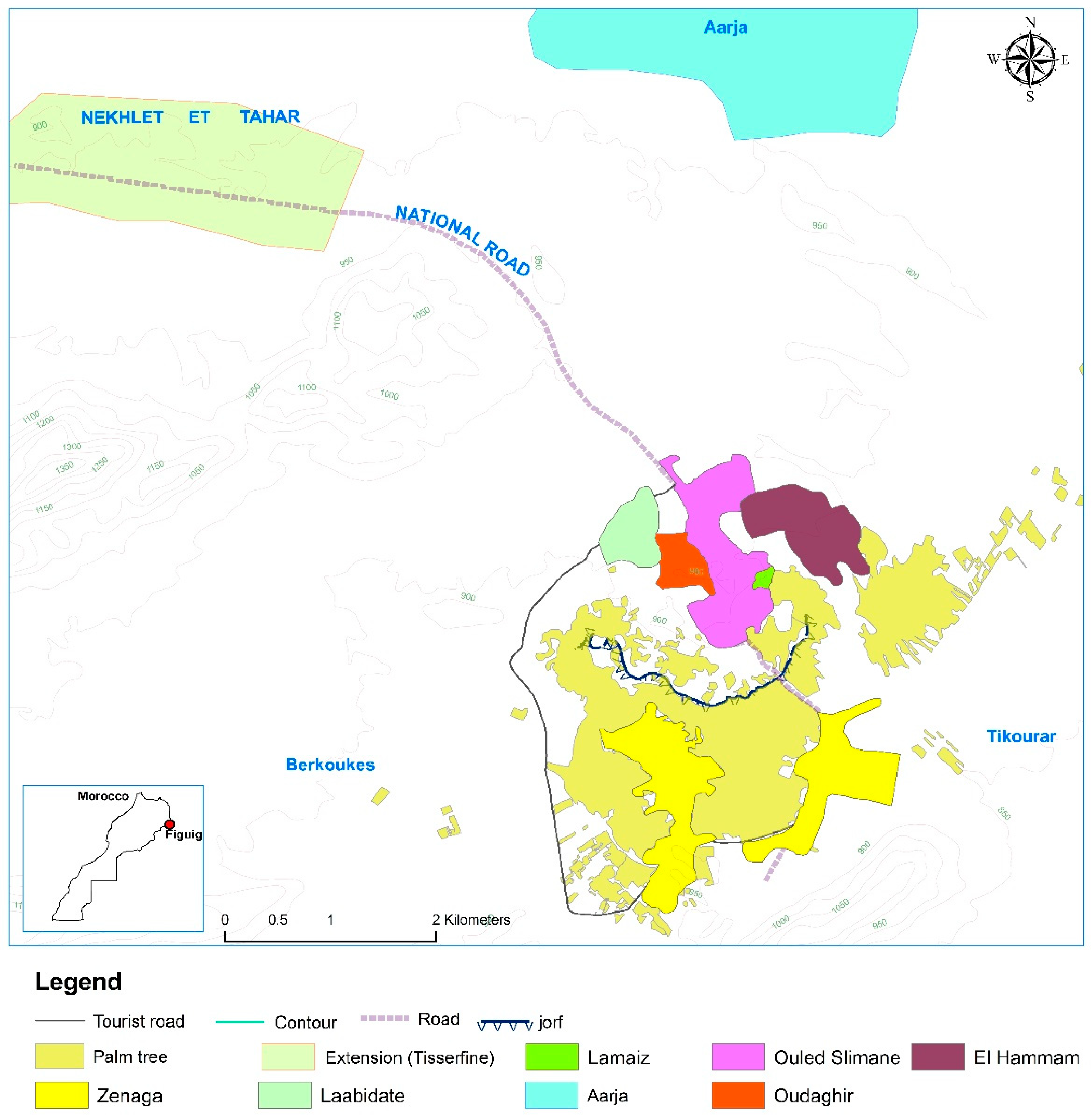
2.1. Study Area and Sampling
2.2. Soil Physicochemical Analyses
2.3. Evaluation of the Number of Infective Propagules of AMF of the Investigated Soils
2.4. Evaluation of AMF Spore Numbers and Identification of AMF Species
2.5. Frequency of Mycorrhization and Intensity of Maize Root Colonization
2.6. Statistical Analyses
3. Results
3.1. Physical and Chemical Soil Properties
3.2. Number of Infective Propagules of AMF in the Soils
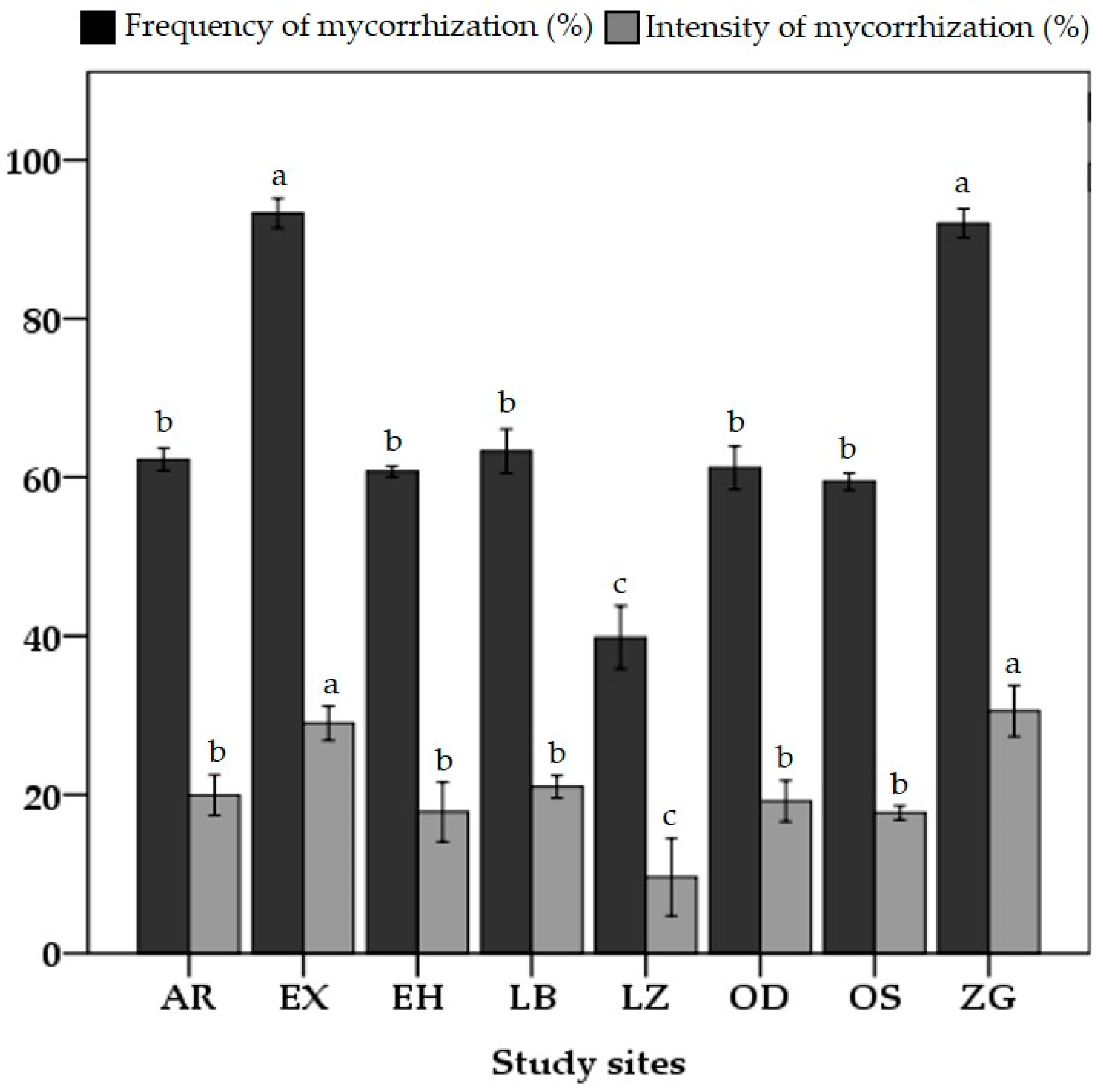
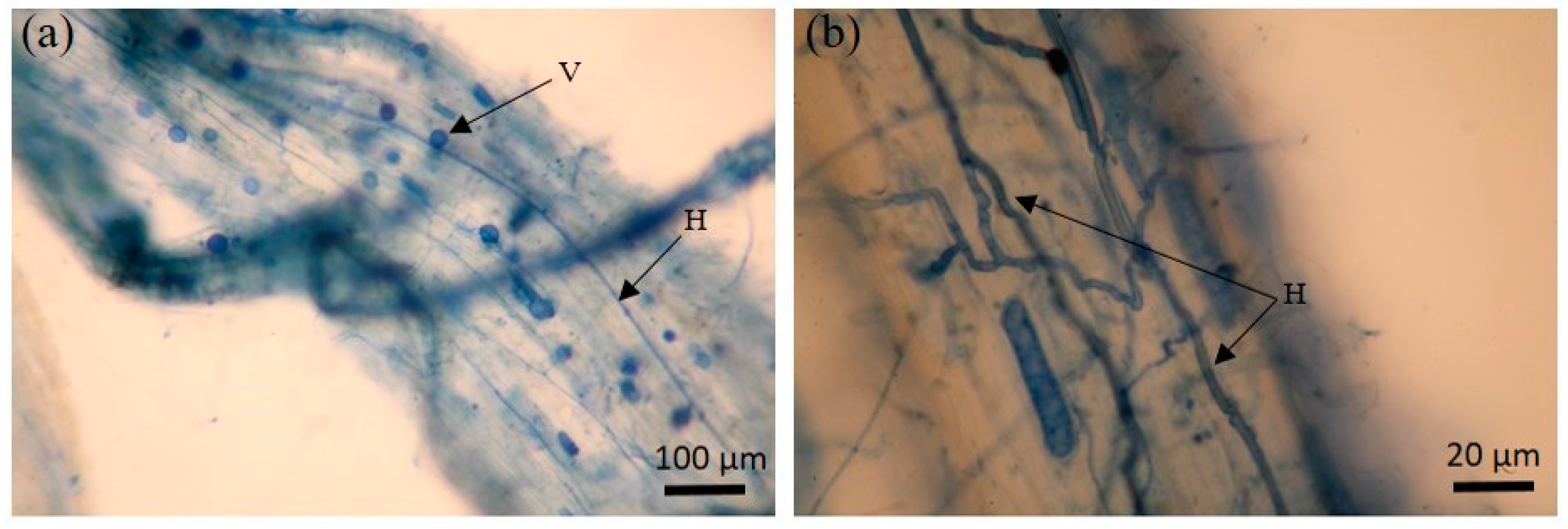
3.3. AMF Root Colonization and Spore Density
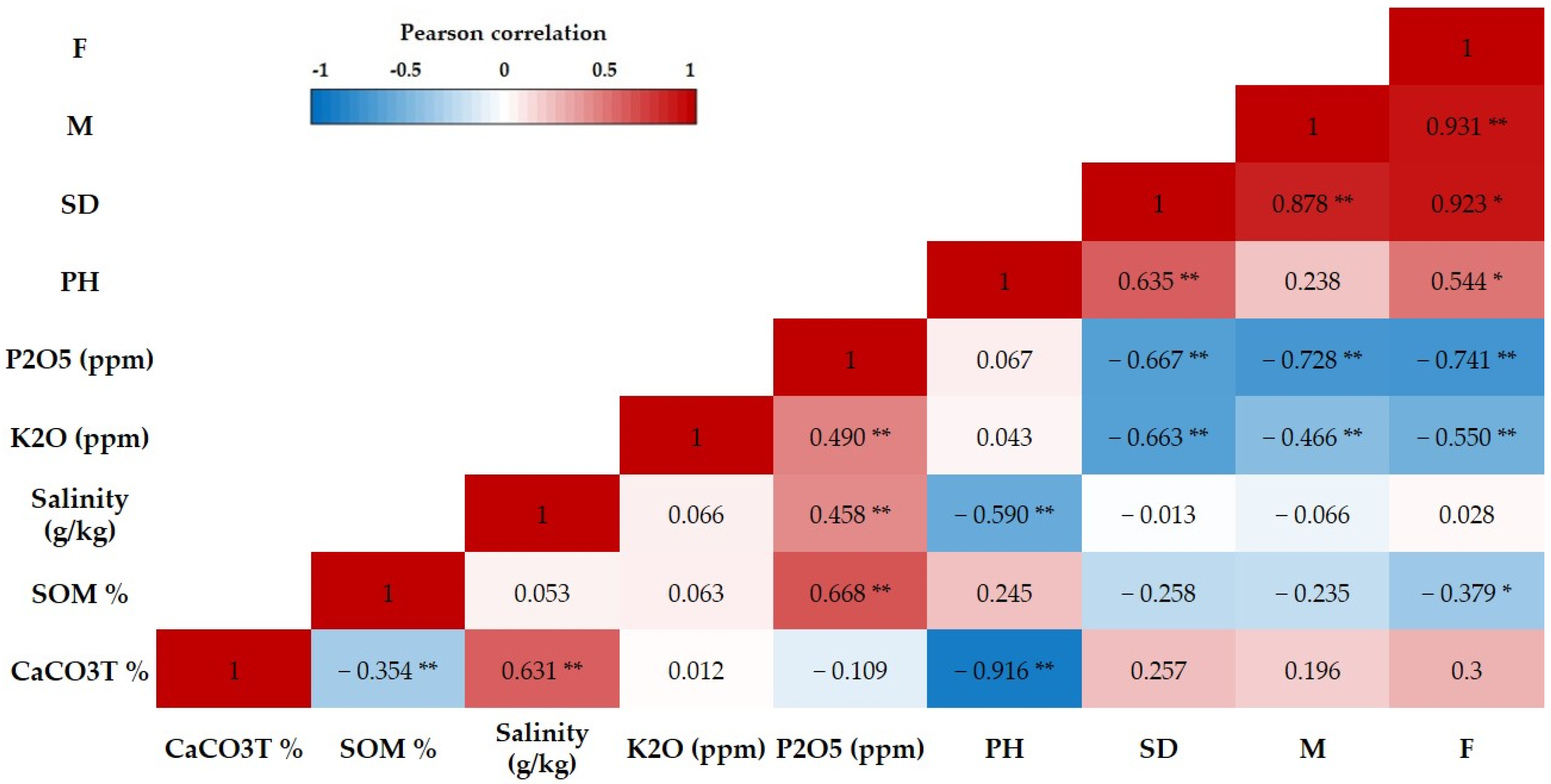
3.4. Correlations among AMF Parameters and Soil Chemical Characteristics
3.5. Morphological Identification of AMF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharif, A.O.; Sanduk, M.; Taleb, H.M. The date palm and its role in reducing soil salinity and global warming. Acta Hortic. 2010, 882, 59–64. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, W.H. Nutritional Quality of 18 Date Fruit Varieties. Int. J. Food Sci. Nutr. 2011, 62, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Obón, C.; Alcaraz, F.; Carreño, E.; Laguna, E.; Amorós, A.; Johnson, D.V.; Díaz, G.; Morte, A. Date Palm Status and Perspective in Spain. In Date Palm Genetic Resources and Utilization; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 489–526. ISBN 978-94-017-9706-1. [Google Scholar]
- Hamza, H.; Jemni, M.; Benabderrahim, M.A.; Mrabet, A.; Touil, S.; Othmani, A.; Salah, M.B. Date Palm Status and Perspective in Tunisia. In Date Palm Genetic Resources and Utilization; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 193–221. ISBN 978-94-017-9693-4. [Google Scholar]
- Chao, C.T.; Krueger, R.R. The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef]
- Verner, D. Tunisia in a Changing Climate: Assessment and Actions for Increased Resilience and Development; A World Bank Study; World Bank: Washington, DC, USA, 2013; ISBN 0-8213-9857-1. [Google Scholar]
- Ghazouani, W.; Marlet, S.; Mekki, I.; Vidal, A. Farmers’ Perceptions and Engineering Approach in the Modernization of a Community-Managed Irrigation Scheme. A Case Study from an Oasis of the Nefzawa (South of Tunisia). Irrig. Drain. 2009, 58, S285–S296. [Google Scholar] [CrossRef]
- Hamed, Y.; Hadji, R.; Redhaounia, B.; Zighmi, K.; Bâali, F.; El Gayar, A. Climate Impact on Surface and Groundwater in North Africa: A Global Synthesis of Findings and Recommendations. Euro Mediterr. J. Environ. Integr. 2018, 3, 25. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Tóth, T.; Ibrahimi, M.-K.; Bouri, S. Effects of Excessive Irrigation of Date Palm on Soil Salinization, Shallow Groundwater Properties, and Water Use in a Saharan Oasis. Environ. Earth Sci. 2017, 76, 590. [Google Scholar] [CrossRef]
- Hachicha, M.; Ben Aissa, I. Managing Salinity in Tunisian Oases. J. Life Sci. 2014, 8, 775–782. [Google Scholar]
- Zeddouk, M. La Problématique Du Développement Agricole Dans Le Milieu Oasien: Cas Du Tafilalet. In Proceedings of the Actes du Symposium International sur le Développement Durable des Systèmes Oasiens, Erfoud, Morocco, 8–10 March 2005; Volume 8, pp. 635–645. [Google Scholar]
- Saaidi, M. Comportement Au Champ de 32 Cultivars de Palmier Dattier Vis-à-Vis Du Bayoud: 25 Années d’observations. Agronomie 1992, 12, 359–370. [Google Scholar] [CrossRef]
- Bouammar, B. Le Développement Agricole Dans Les Régions Sahariennes Etude de Cas de La Région de Ouargla et de La Région de Biskra (2006–2008). Ph.D. Thesis, Université Kasdi Merbah de Ouargla, Ouargla, Algeria, 2010. [Google Scholar]
- Abohatem, M.; Zouine, J.; El Hadrami, I. Low Concentrations of BAP and High Rate of Subcultures Improve the Establishment and Multiplication of Somatic Embryos in Date Palm Suspension Cultures by Limiting Oxidative Browning Associated with High Levels of Total Phenols and Peroxidase Activities. Sci. Hortic. 2011, 130, 344–348. [Google Scholar] [CrossRef]
- Newsham, K.K.; Fitter, A.H.; Watkinson, A.R. Arbuscular Mycorrhiza Protect an Annual Grass from Root Pathogenic Fungi in the Field. J. Ecol. 1995, 83, 991–1000. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water Relations, Drought and Vesicular-Arbuscular Mycorrhizal Symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Neumann, E.; George, E. Colonisation with the Arbuscular Mycorrhizal Fungus Glomus Mosseae (Nicol. & Gerd.) Enhanced Phosphorus Uptake from Dry Soil in Sorghum bicolor (L.). Plant Soil 2004, 261, 245–255. [Google Scholar]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and Soil Structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Cui, M.; Nobel, P.S. Nutrient Status, Water Uptake and Gas Exchange for Three Desert Succulents Infected with Mycorrhizal Fungi. New Phytol. 1992, 122, 643–649. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of Mycorrhizal Fungi as a Strategy for Improving the Drought Tolerance in Date Palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Duponnois, R.; Ramanankierana, H.; Hafidi, M.; Baohanta, R.; Baudoin, E.; Thioulouse, J.; Sanguin, H.; Ba, A.; Galiana, A.; Bally, R. Native Plant Resources to Optimize the Performances of Forest Rehabilitation in Mediterranean and Tropical Environment: Some Examples of Nursing Plant Species That Improve the Soil Mycorrhizal Potential. C. R. Biol. 2013, 336, 265–272. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcón, R. Drought Tolerance and Antioxidant Activities in Lavender Plants Colonized by Native Drought-Tolerant or Drought-Sensitive Glomus Species. Microb. Ecol. 2007, 54, 543–552. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Rohr, J.R.; Aldrich-Wolfe, L.; Morton, J.B. Role of Niche Restrictions and Dispersal in the Composition of Arbuscular Mycorrhizal Fungal Communities. J. Ecol. 2007, 95, 95–105. [Google Scholar] [CrossRef]
- Antunes, P.M.; Koch, A.M.; Morton, J.B.; Rillig, M.C.; Klironomos, J.N. Evidence for Functional Divergence in Arbuscular Mycorrhizal Fungi from Contrasting Climatic Origins. New Phytol. 2011, 189, 507–514. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA) Press: Washington, DC, USA, 2005. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Mathieu, C.; Pieltain, F.; Jeanroy, E. Analyse Chimique des Sols: Méthodes Choisies; Tec & Doc/Lavoisier: Paris, France, 2003; 408p, ISBN 2-7430-0620-X. [Google Scholar]
- He, Y.; DeSutter, T.; Prunty, L.; Hopkins, D.; Jia, X.; Wysocki, D.A. Evaluation of 1:5 Soil to Water Extract Electrical Conductivity Methods. Geoderma 2012, 185–186, 12–17. [Google Scholar] [CrossRef]
- P94-048; Soil: Investigation and Testing—Determination of the Carbonate Content—Calcimeter Method. AFNOR: Paris, France, 2002.
- Ritchey, E.L.; Mcgrath, J.M.; Gehring, D. Determining Soil Texture by Feel; Agriculture and Natural Resources Publication 139; University of Kentucky, College of Agriculture, Food and Environment: Lexington, KY, USA, 2015. [Google Scholar]
- Plenchette, C.; Perrin, R.; Duvert, P. The Concept of Soil Infectivity and a Method for Its Determination as Applied to Endomycorrhizas. Can. J. Bot. 1989, 67, 112–115. [Google Scholar] [CrossRef]
- Liu, R.; Wang, F. Selection of Appropriate Host Plants Used in Trap Culture of Arbuscular Mycorrhizal Fungi. Mycorrhiza 2003, 13, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Fisher, R.A.; Yates, F. Statistical Tables for Biological Agriculture and Medical Research, 6th ed.; Hafner Publishing Company: Davien, CT, USA, 1970. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Koskey, R.E. A Convenient, Permanent Slide Mounting Medium. Newsl. Mycol. Soc. Am. 1983, 34, 59. [Google Scholar]
- Brundrett, M. Practical Methods in Mycorrhiza Research: Based on a Workshop Organized in Conjunction with the Ninth North American Conference on Mycorrhizae; University of Guelph: Guelph, ON, Canada, 1994. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Trouvelot, A. Mesure Du Taux de Mycorrhization d’un Systeme Radiculaire. Recherche de Methods d’estimation Ayant Une Signification Fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sci. Med. Res. 2011, 21, 30. [Google Scholar]
- Oyediran, O.K.; Kumar, A.G.; Neelam, J. Arbuscular Mycorrhizal Fungi Associated with Rhizosphere of Tomato Grown in Arid and Semi-Arid Regions of Indian Desert. Asian J. Agric. Res. 2018, 12, 10–18. [Google Scholar] [CrossRef]
- Marschner, H.; Cakmak, I. Mechanism of Phosphorus-induced Zinc Deficiency in Cotton. II. Evidence for Impaired Shoot Control of Phosphorus Uptake and Translocation under Zinc Deficiency. Physiol. Plant. 1986, 68, 491–496. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Soil Factors in Relation to Distribution and Occurrence of Vesicular Arbuscular Mycorrhiza. In Techniques in Mycorrhizal Studies; Mukerji, K.G., Manoharachary, C., Chamola, B.P., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Lagrange, A. Etudes Écologique et Microbiologique des Espèces du Genre Costularia (Cyperaceae) Pionnières des Sols Ultramafiques de Nouvelle-Calédonie: Applications à La Restauration Écologique. Ph.D. Thesis, Université de Nouvelle Calédonie, Nouméa, France, 2009. [Google Scholar]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil Type and Land Use Intensity Determine the Composition of Arbuscular Mycorrhizal Fungal Communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Bhat, B.A.; Sheikh, M.A.; Tiwari, A. J Presearch ARTICLE. Int. J. Plant Sci. 2014, 9, 1–6. [Google Scholar]
- Toh, S.; Lihan, S.; Yong, C.; Tiang, B.; Rakiya, A.; Edward, R. Isolation and Characterisation of Arbuscular Mycorrhizal (AM) Fungi Spores from Selected Plant Roots and Their Rhizosphere Soil Environment. Malays. J. Microbiol. 2018, 14, 335–343. [Google Scholar]
- Gai, J.; Liu, R. Effects of Soil Factors on Arbuscular Mycorrhizae (AM) Fungi around Roots of Wild Plants. J. Appl. Ecol. 2003, 14, 470–472. [Google Scholar]
- Gunasekaran, P.; Sundaresan, P.; Raja, N.U.; Lakshmanan, M. Effect of PH, Temperature and Nutrients on the Germination of a Vesicular-Arbuscular Mycorrhizal Fungus, Glomus Fasciculatum In Vitro. Proc. Plant Sci. 1987, 97, 231–234. [Google Scholar] [CrossRef]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and Temporal Structuring of Arbuscular Mycorrhizal Communities Is Differentially Influenced by Abiotic Factors and Host Crop in a Semi-Arid Prairie Agroecosystem. FEMS Microbiol. Ecol. 2014, 88, 333–344. [Google Scholar] [CrossRef]
- Mosbah, M.; Philippe, D.L.; Mohamed, M. Molecular Identification of Arbuscular Mycorrhizal Fungal Spores Associated to the Rhizosphere of Retama Raetam in Tunisia. Soil Sci. Plant Nutr. 2018, 64, 335–341. [Google Scholar] [CrossRef]
- Melo, C.D.; Luna, S.; Krüger, C.; Walker, C.; Mendonça, D.; Fonseca, H.M.; Jaizme-Vega, M.; da Câmara Machado, A. Arbuscular Mycorrhizal Fungal Community Composition Associated with Juniperus Brevifolia in Native Azorean Forest. Acta Oecol. 2017, 79, 48–61. [Google Scholar] [CrossRef]
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L. Arbuscular Mycorrhizal Fungi Associated with Phoenix dactylifera L. Grown in Tunisian Sahara Oases of Different Salinity Levels. Symbiosis 2020, 81, 173–186. [Google Scholar] [CrossRef]
- Symanczik, S.; Błaszkowski, J.; Koegel, S.; Boller, T.; Wiemken, A.; Al-Yahya’Ei, M.N. Isolation and Identification of Desert Habituated Arbuscular Mycorrhizal Fungi Newly Reported from the Arabian Peninsula. J. Arid Land 2014, 6, 488–497. [Google Scholar] [CrossRef]



| Site/Soil Property | ZG | OD | LZ | OS | EH | LB | AR | EX |
|---|---|---|---|---|---|---|---|---|
| pH | 7.8 | 7.6 | 7.4 | 7.7 | 7.7 | 7.6 | 7.7 | 8.1 |
| SOM (%) | 0.4 | 0.4 | 1.8 | 0.9 | 1 | 1.7 | 0.6 | 0.4 |
| P2O5 (ppm) | 20.3 | 37.4 | 100 | 78.6 | 36.3 | 72 | 24.1 | 23.3 |
| K2O (ppm) | 53 | 193.5 | 158.3 | 176 | 211 | 158.3 | 70.4 | 105.5 |
| CaCO3T (%) | 9.5 | 18.5 | 22 | 21 | 20 | 21 | 8 | 45 |
| Salinity (g/kg) | 0.1 | 0.2 | 0.7 | 1.1 | 0.1 | 0.9 | 0.3 | 1.2 |
| Texture | Clay loam | Clay loam | Clay loam | Clay loam | Clay loam | Clay loam | Sandy clay loam | Sandy clay loam |
| Repetition ×5 | |||||||
|---|---|---|---|---|---|---|---|
| Dilution | 1 | 1/4 | 1/16 | 1/64 | 1/256 | 1/1024 | |
| Site | |||||||
| ZG | 5 | 5 | 5 | 5 | 5 | 5 | |
| OD | 5 | 5 | 5 | 5 | 3 | 3 | |
| LZ | 5 | 5 | 5 | 5 | 3 | 2 | |
| OS | 5 | 5 | 5 | 5 | 5 | 3 | |
| EH | 5 | 5 | 5 | 5 | 4 | 3 | |
| LB | 5 | 5 | 5 | 5 | 3 | 3 | |
| EX | 5 | 5 | 5 | 5 | 5 | 5 | |
| AR | 5 | 5 | 5 | 5 | 4 | 3 | |
| ID | Size | Color | External Wall | Internal Wall | Suspension Hyphae | Abundance | Form | Internal Structures | Wall Number | Single-Spore or Clustered |
|---|---|---|---|---|---|---|---|---|---|---|
| FIG1 | 150 µm | Yellow–brown | Present | Present | Present | Mediocre | Globular | Lipid droplets | 3 | In a cluster |
| FIG2 | 120 µm | Brown | Present | Present | Present | Mediocre | Globular | Lipid droplets | 2 | Single |
| FIG3 | 50 µm | Orange | Present | Present | Present | High | Spherical | Lipid droplets | 2 | Single |
| FIG4 | 100 µm | Yellow–brown | Present | Absent | Present | Mediocre | Subglobular | Lipid droplets | 2 | In a cluster |
| FIG5 | 300 µm | Orange yellow | Present | Present | Absent | High | Oval | Lipid droplets | 3 | In a cluster |
| FIG6 | 100 µm | Yellow | Present | Present | Present | High | Subglobular | Lipid droplets | 3 | In a cluster |
| FIG7 | 250 µm | Orange | Present | Absent | Absent | Rare | Globular | Lipid droplets | 2 | Single |
| FIG8 | 130 µm | Red–brown | Present | Present | Present | Mediocre | Globular | Lipid droplets | 3 | Single |
| FIG9 | 100 µm | Brown | Present | Present | Present | High | Oval | Lipid droplets | 3 | In a cluster |
| FIG10 | 120 µm | Light yellow | Present | Present | Absent | Mediocre | Globular | Lipid droplets | 2 | Single |
| FIG 11 | 270 µm | Yellow | Present | Present | Present | Mediocre | Subglobular | Lipid droplets | 2 | In a cluster |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagou, E.; Chakroune, K.; Abbas, M.; Lamkami, T.; Hakkou, A. Evaluation of the Mycorrhizal Potential of Date Palm (Phoenix dactylifera L.) Rhizosphere Soils in the Figuig Oasis (Southeastern Morocco). J. Fungi 2023, 9, 931. https://doi.org/10.3390/jof9090931
Gagou E, Chakroune K, Abbas M, Lamkami T, Hakkou A. Evaluation of the Mycorrhizal Potential of Date Palm (Phoenix dactylifera L.) Rhizosphere Soils in the Figuig Oasis (Southeastern Morocco). Journal of Fungi. 2023; 9(9):931. https://doi.org/10.3390/jof9090931
Chicago/Turabian StyleGagou, Elmostafa, Khadija Chakroune, Mahmoud Abbas, Touria Lamkami, and Abdelkader Hakkou. 2023. "Evaluation of the Mycorrhizal Potential of Date Palm (Phoenix dactylifera L.) Rhizosphere Soils in the Figuig Oasis (Southeastern Morocco)" Journal of Fungi 9, no. 9: 931. https://doi.org/10.3390/jof9090931






