Evaluation of Different Gene Prediction Tools in Coccidioides immitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomic Data
2.2. Previously Published Data
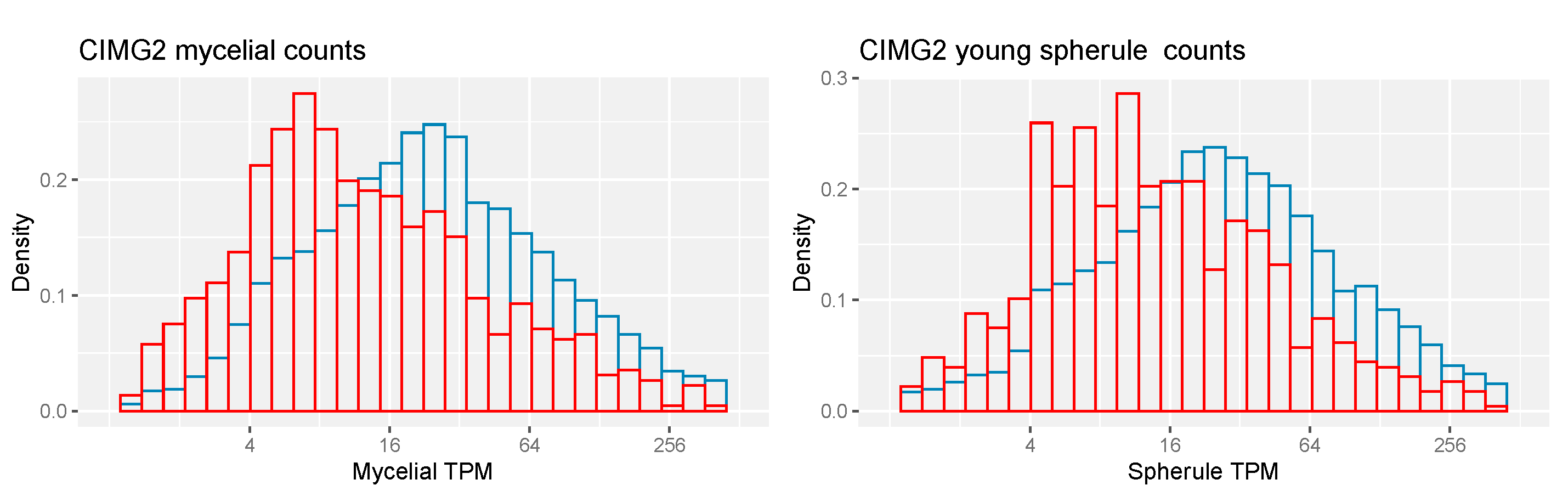
2.3. CIMG2 and Augustus Annotations
2.4. Software
3. Results
3.1. Comparing All CIMG-Predicted Genes to CIMG-Unique Genes
3.2. Comparing All CIMG2-Predicted Genes to CIMG2-Unique Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weisman, C.M.; Murray, A.W.; Eddy, S.R. Many, but not all, lineage-specific genes can be explained by homology detection failure. PLoS Biol. 2020, 18, e3000862. [Google Scholar] [CrossRef] [PubMed]
- Weisman, C.M.; Murray, A.W.; Eddy, S.R. Mixing genome annotation methods in a comparative analysis inflates the apparent number of lineage-specific genes. Curr. Biol. 2022, 32, 2632–2639.e2. [Google Scholar] [CrossRef] [PubMed]
- Yandell, M.; Ence, D. A beginner’s guide to eukaryotic genome annotation. Nat. Rev. Genet. 2012, 13, 329–342. [Google Scholar] [CrossRef]
- McHardy, A.C. Finding Genes in Genome Sequence. In Bioinformatics: Data, Sequence Analysis and Evolution; Keith, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 163–177. [Google Scholar]
- Scalzitti, N.; Jeannin-Girardon, A.; Collet, P.; Poch, O.; Thompson, J.D. A benchmark study of ab initio gene prediction methods in diverse eukaryotic organisms. BMC Genom. 2020, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Li, Y. A brief review of computational gene prediction methods. Genom. Proteom. Bioinform. 2004, 2, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Schoffmann, O.; Morgenstern, B.; Waack, S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinform. 2006, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Korf, I. Gene finding in novel genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef]
- Testa, A.C.; Hane, J.K.; Ellwood, S.R.; Oliver, R.P. CodingQuarry: Highly accurate hidden Markov model gene prediction in fungal genomes using RNA-seq transcripts. BMC Genom. 2015, 16, 170. [Google Scholar] [CrossRef]
- Ter-Hovhannisyan, V.; Lomsadze, A.; Chernoff, Y.O.; Borodovsky, M. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 2008, 18, 1979–1990. [Google Scholar] [CrossRef]
- de Melo Teixeira, M.; Stajich, J.E.; Sahl, J.W.; Thompson, G.R.; Brem, R.B.; Dubin, C.A.; Blackmon, A.V.; Mead, H.L.; Keim, P.; Barker, B.M. A chromosomal-level reference genome of the widely utilized Coccidioides posadasii laboratory strain “Silveira”. G3 2022, 12, jkac031. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Barker, B.M.; Stajich, J.E. Improved Reference Genome Sequence of Coccidioides immitis Strain WA_211, Isolated in Washington State. Microbiol. Resour. Announc. 2019, 8, e00149-19. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.A.; Ross, B.S.; Cramer, R.A.; Stajich, J.E. The pan-genome of Aspergillus fumigatus provides a high-resolution view of its population structure revealing high levels of lineage-specific diversity driven by recombination. PLoS Biol. 2022, 20, e3001890. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Stevens, D.A.; Hung, C.Y.; Beyhan, S.; Taylor, J.W.; Shubitz, L.F.; Duttke, S.H.; Heidari, A.; Johnson, R.H.; Deresinski, S.C.; et al. Coccidioides Species: A Review of Basic Research: 2022. J. Fungi 2022, 8, 859. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef]
- Thompson, G.R.; Ampel, N.M.; Blair, J.E.; Donovan, F.; Fierer, J.; Galgiani, J.N.; Heidari, A.; Johnson, R.; Shatsky, S.A.; Uchiyama, C.M.; et al. Controversies in the Management of Central Nervous System Coccidioidomycosis. Clin. Infect. Dis. 2022, 75, 555–559. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Stajich, J.E.; Park, D.J.; Whiston, E.; Hung, C.Y.; McMahan, C.; White, J.; Sykes, S.; et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Carlin, A.F.; Beyhan, S.; Peña, J.F.; Stajich, J.E.; Viriyakosol, S.; Fierer, J.; Kirkland, T.N. Transcriptional Analysis of Coccidioides immitis Mycelia and Spherules by RNA Sequencing. JoF 2021, 7, 366. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Muszewska, A.; Stajich, J.E. Analysis of Transposable Elements in Coccidioides Species. J. Fungi 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Duttke, S.H.; Beyhan, S.; Singh, R.; Neal, S.; Viriyakosol, S.; Fierer, J.; Kirkland, T.N.; Stajich, J.E.; Benner, C.; Carlin, A.F. Decoding Transcription Regulatory Mechanisms Associated with Coccidioides immitis Phase Transition Using Total RNA. mSystems 2022, 7, e0140421. [Google Scholar] [CrossRef] [PubMed]
- Stajich, J. Stajichlab/Coccidioides_immitis_RS_reannotation: Dataset freeze for Kirkland et al. (v1.0.0). Zenodo 2023. [Google Scholar] [CrossRef]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res 2020, 9, 304. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Steinegger, M.; Soding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Goulet, K.M.; Storfie, E.R.M.; Saville, B.J. Exploring links between antisense RNAs and pathogenesis in Ustilago maydis through transcript and gene characterization. Fungal Genet. Biol. 2020, 134, 103283. [Google Scholar] [CrossRef]
- Hongay, C.F.; Grisafi, P.L.; Galitski, T.; Fink, G.R. Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell 2006, 127, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Cemel, I.A.; Ha, N.; Schermann, G.; Yonekawa, S.; Brunner, M. The coding and noncoding transcriptome of Neurospora crassa. BMC Genom. 2017, 18, 978. [Google Scholar] [CrossRef] [PubMed]
- Venters, B.J.; Pugh, B.F. How eukaryotic genes are transcribed. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 117–141. [Google Scholar] [CrossRef]
- Nevers, A.; Doyen, A.; Malabat, C.; Neron, B.; Kergrohen, T.; Jacquier, A.; Badis, G. Antisense transcriptional interference mediates condition-specific gene repression in budding yeast. Nucleic Acids Res. 2018, 46, 6009–6025. [Google Scholar] [CrossRef] [PubMed]
- Whiston, E.; Zhang Wise, H.; Sharpton, T.J.; Jui, G.; Cole, G.T.; Taylor, J.W. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS ONE 2012, 7, e41034. [Google Scholar] [CrossRef]
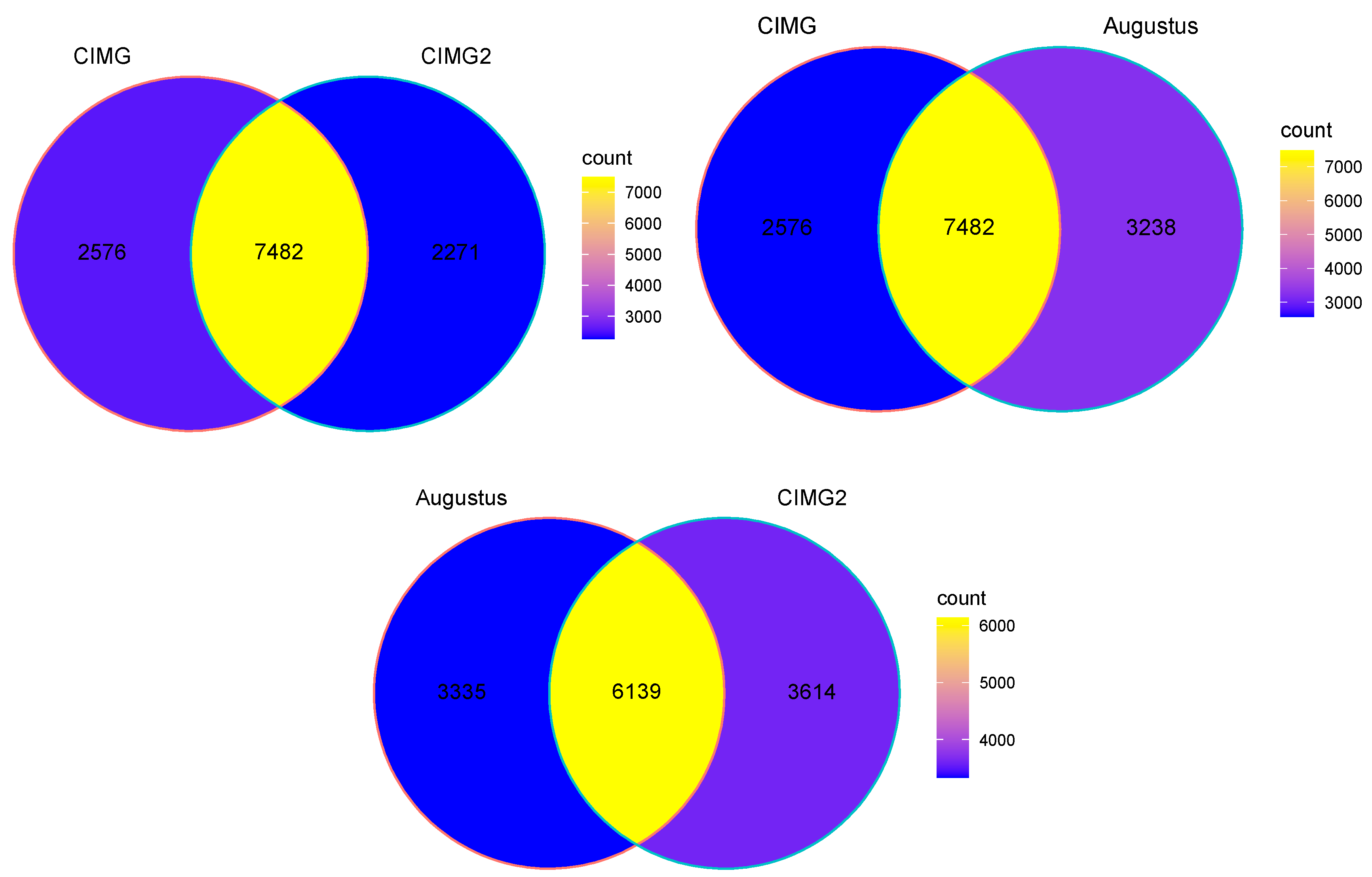
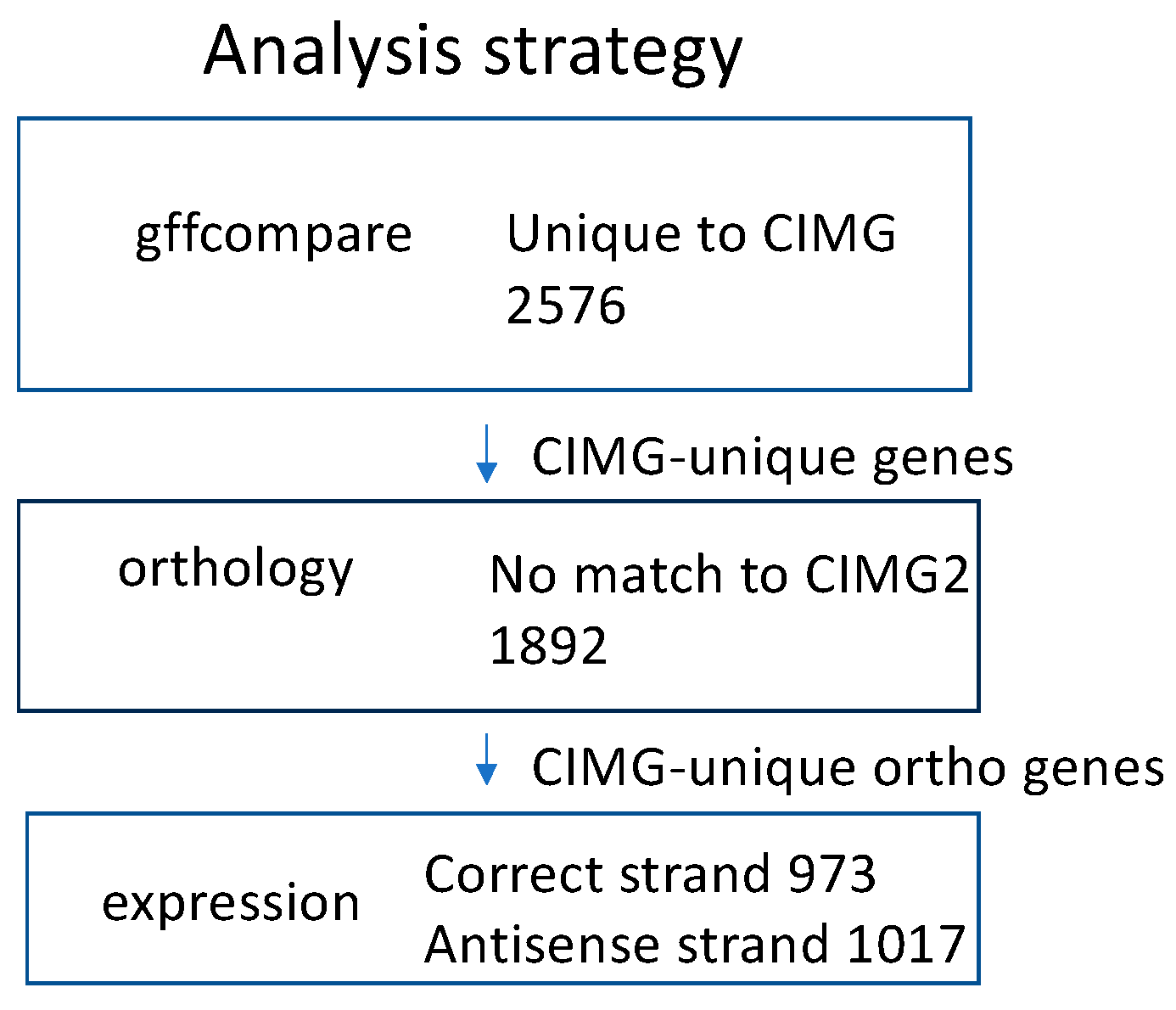

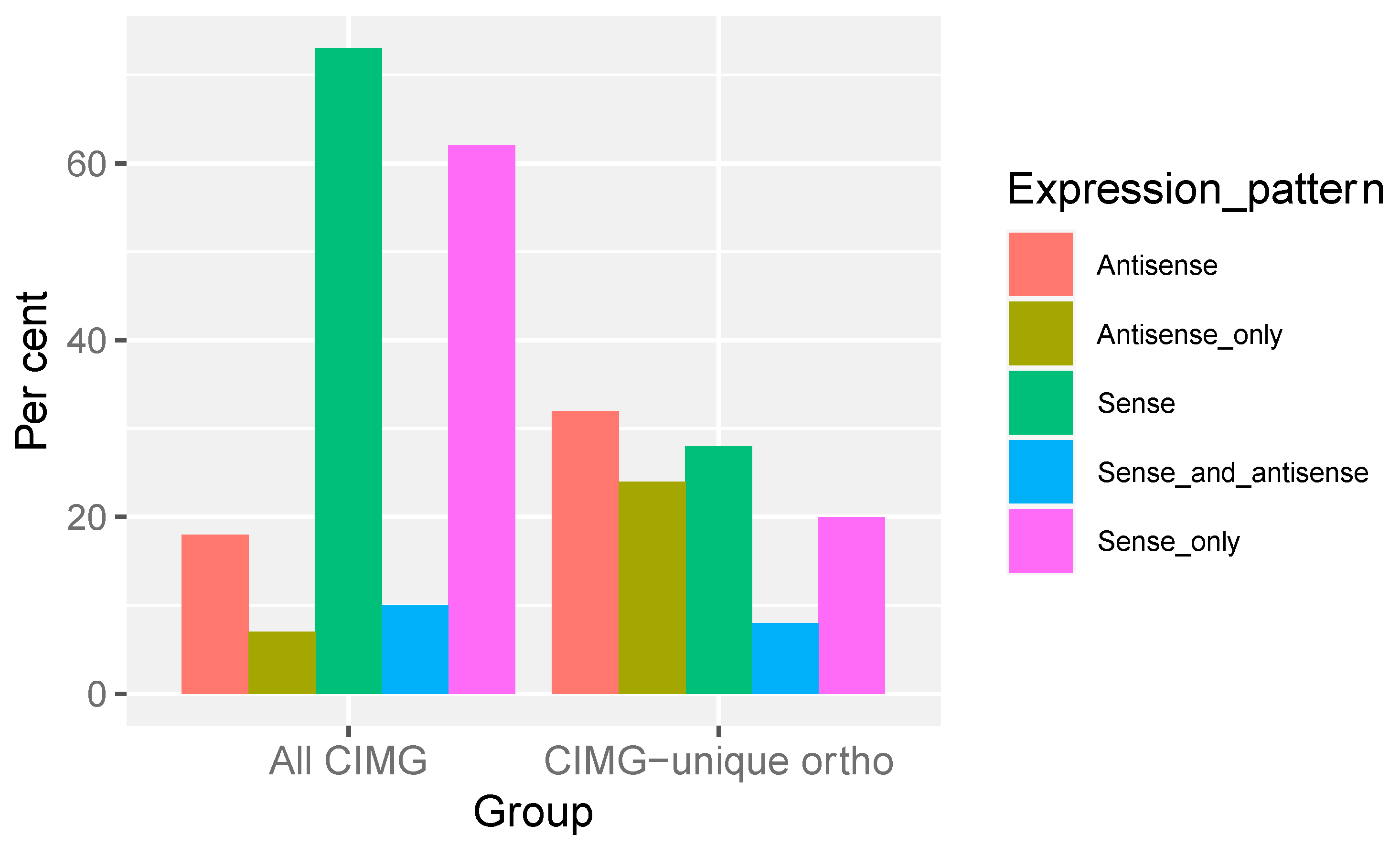

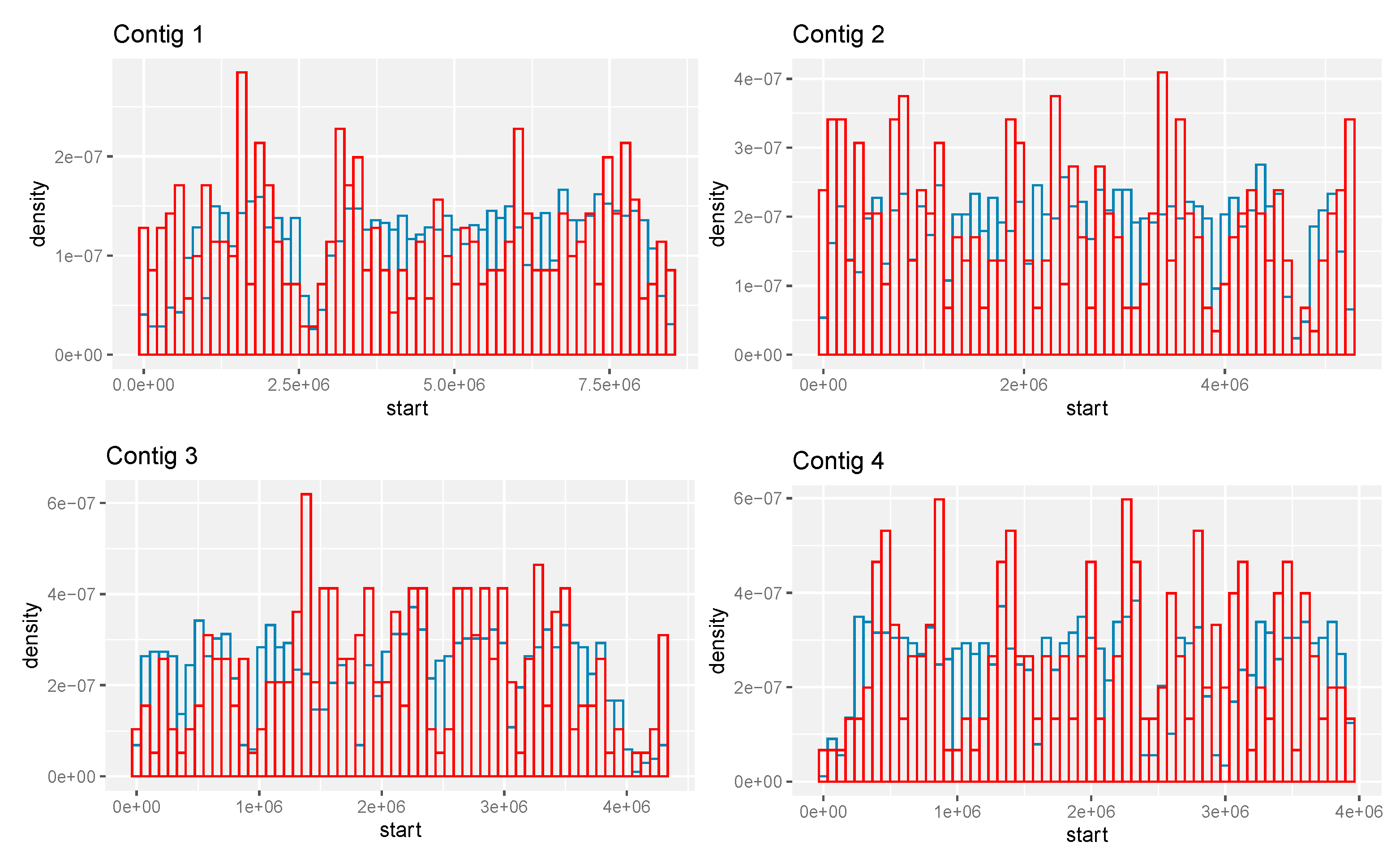
| Total | % a | CIMG-Unique | % b | CIMG-Unique Ortho | % c | |
|---|---|---|---|---|---|---|
| Total | 10,058 | 2576 | 1892 | |||
| Hypothetical product description | 3318 | 32.99 | 1905 | 73.95 | 1645 | 86.95 |
| Any PFAM domain | 6077 | 60.42 | 523 | 20.30 | 63 | 3.33 |
| Molecular function GO annotation | 4449 | 44.23 | 407 | 15.80 | 38 | 2.01 |
| Linage-specific (< 10 orthologs) | 2381 | 23.67 | 1741 | 67.59 | 1589 | 83.99 |
| Number of orthologs d | 204 | 2 | 1 | |||
| Genes with homologs e | 6388 | 63.51 | 518 | 20.11 | 30 | 1.59 |
| Differential expression f | 2009 | 19.97 | 581 | 22.55 | 406 | 21.46 |
| Protein length g | 347 | 155 | 131 |
| % a | CIMG2-Unique | % b | CIMG2-Unique Ortho | % c | ||
|---|---|---|---|---|---|---|
| Total | 9753 | 2271 | 1066 | |||
| “Hypothetical protein” product description | 7541 | 77.32 | 1988 | 87.54 | 959 | 89.96 |
| Any PFAM domain | 6425 | 65.88 | 918 | 40.42 | 301 | 28.24 |
| Genes with homologs d | 8712 | 89.33 | 740 | 32.58 | 325 | 30.49 |
| Protein length e | 389 | 168 | 137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkland, T.N.; Beyhan, S.; Stajich, J.E. Evaluation of Different Gene Prediction Tools in Coccidioides immitis. J. Fungi 2023, 9, 1094. https://doi.org/10.3390/jof9111094
Kirkland TN, Beyhan S, Stajich JE. Evaluation of Different Gene Prediction Tools in Coccidioides immitis. Journal of Fungi. 2023; 9(11):1094. https://doi.org/10.3390/jof9111094
Chicago/Turabian StyleKirkland, Theo N., Sinem Beyhan, and Jason E. Stajich. 2023. "Evaluation of Different Gene Prediction Tools in Coccidioides immitis" Journal of Fungi 9, no. 11: 1094. https://doi.org/10.3390/jof9111094






