Endophytic Fungus Negatively Affects Salt Tolerance of Tall Fescue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Greenhouse
2.1.2. Common Garden
2.2. Field Populations
2.3. Statistical Analysis
2.3.1. Greenhouse and Common Garden
2.3.2. Field Populations
3. Results
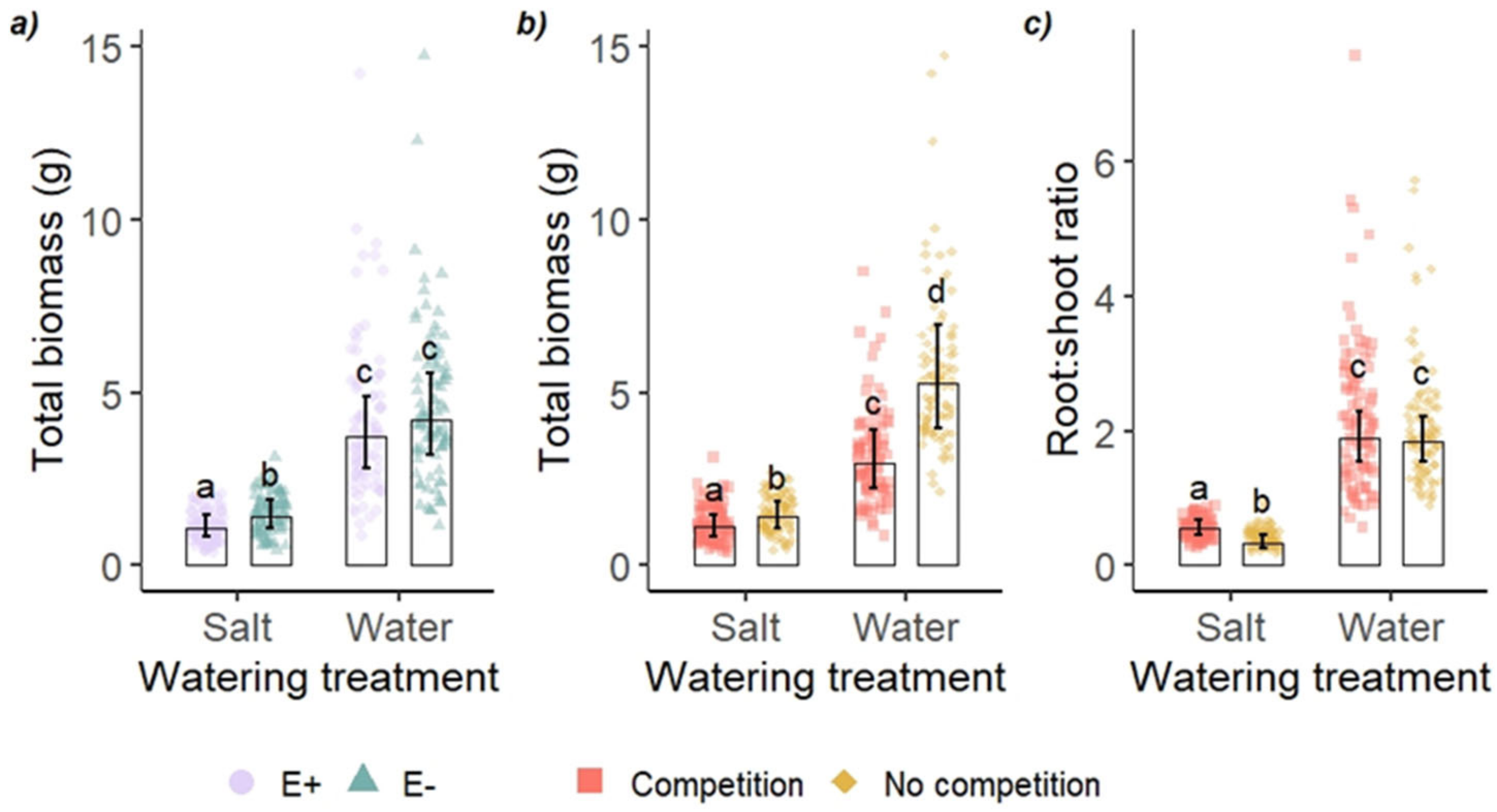
3.1. Greenhouse
3.2. Common Garden
3.3. Field Populations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO and ITPS. Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015; p. 607. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Kuldau, G.; Bacon, C. Clavicipitaceous Endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control. 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Faeth, S.H. Ecology and Evolution of the Grass-Endophyte Symbiosis; Oxford University Press: New York, NY, USA, 2009; ISBN 978-0-19-530808-2. [Google Scholar]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal endophyte Epichloë bromicola infection regulates anatomical changes to account for salt stress tolerance in wild barley (Hordeum brevisubulatum). Plant Soil 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Pereira, E.C.; Vazquez de Aldana, B.R.; Arellano, J.B.; Zabalgogeazcoa, I. The role of fungal microbiome components on the adaptation to salinity of Festuca rubra subsp. pruinosa. Front. Plant Sci. 2021, 12, 695717. [Google Scholar] [CrossRef]
- Ahlholm, J.U.; Helander, M.; Lehtimäki, S.; Wäli, P.; Saikkonen, K. Vertically transmitted fungal endophytes: Different responses of host-parasite systems to environmental conditions. Oikos 2002, 99, 173–183. [Google Scholar] [CrossRef]
- Lehtonen, P.; Helander, M.; Saikkonen, K. Are endophyte-mediated effects on herbivores conditional on soil nutrients? Oecologia 2005, 142, 38–45. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte–grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef]
- Saikkonen, K.; Phillips, T.D.; Faeth, S.H.; McCulley, R.L.; Saloniemi, I.; Helander, M. Performance of endophyte infected tall fescue in Europe and North America. PLoS ONE 2016, 11, e0157382. [Google Scholar] [CrossRef] [Green Version]
- Clay, K.; Holah, J. Fungal endophyte symbiosis and plant diversity in successional fields. Science 1999, 285, 1742–1744. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef]
- Saikkonen, K. Kentucky 31, far from home. Science 2000, 287, 1887. [Google Scholar] [CrossRef] [Green Version]
- Clay, K.; Marks, S.; Cheplick, G.P. Effects of insect herbivory and fungal endophyte infection on competitive interactions among grasses. Ecology 1993, 74, 1767–1777. [Google Scholar] [CrossRef]
- Clay, K. Fungal endophytes, herbivores and the structure of grassland communities. In Multitrophic Interactions in Terrestrial Systems; Blackwell: Oxford, UK, 1997; pp. 151–169. ISBN 978-0-521-83995-2. [Google Scholar]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.-H.; Foster, K.J.; et al. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef] [Green Version]
- Saikkonen, K.; Ahlholm, J.; Helander, M.; Lehtimäki, S.; Niemeläinen, O. Endophytic fungi in wild and cultivated grasses in Finland. Ecography 2000, 23, 360–366. [Google Scholar] [CrossRef]
- Saari, S.; Helander, M.; Faeth, S.H.; Saikkonen, K. The effects of endophytes on seed production and seed predation of tall fescue and meadow fescue. Microb. Ecol. 2010, 60, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wäli, P.R.; Ahlholm, J.U.; Helander, M.; Saikkonen, K. Occurrence and genetic structure of the systemic grass endophyte Epichloë festucae in fine fescue populations. Microb. Ecol. 2007, 53, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dirihan, S.; Helander, M.; Väre, H.; Gundel, P.E.; Garibaldi, L.A.; Irisarri, J.G.N.; Saloniemi, I.; Saikkonen, K. Geographic variation in Festuca rubra L. ploidy levels and systemic fungal endophyte frequencies. PloS ONE 2016, 11, e0166264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronstein, J.L. The exploitation of mutualisms. Ecol. Lett. 2001, 4, 277–287. [Google Scholar] [CrossRef]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Gundel, P.E.; Batista, W.B.; Texeira, M.; Martínez-Ghersa, M.A.; Omacini, M.; Ghersa, C.M. Neotyphodium Endophyte infection frequency in annual grass populations: Relative importance of mutualism and transmission efficiency. Proc. R. Soc. B Biol. Sci. 2008, 275, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Clay, K.; Holah, J.; Rudgers, J.A. Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc. Natl. Acad. Sci. USA 2005, 102, 12465–12470. [Google Scholar] [CrossRef] [Green Version]
- Lembicz, M.; Olejniczak, P. The fungus Epichloë typhina in populations of a halophyte Puccinellia distans: Salinity as a possible inhibitor of infection. Acta Soc. Bot. Pol. 2009, 78, 81–86. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Mirlohi, A. Neotyphodium endophytes trigger salt resistance in tall and meadow fescues. J. Plant Nutr. Soil Sci. 2010, 173, 952–957. [Google Scholar] [CrossRef]
- Yin, L.; Ren, A.; Wei, M.; Wu, L.; Zhou, Y.; Li, X.; Gao, Y. Neotyphodium coenophialum-infected tall fescue and its potential application in the phytoremediation of saline soils. Int. J. Phytoremediation 2014, 16, 235–246. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Mattingly, W.B.; Koslow, J.M. Mutualistic fungus promotes plant invasion into diverse communities. Oecologia 2005, 144, 463–471. [Google Scholar] [CrossRef]
- Marks, S.; Clay, K.; Cheplick, G.P. Effects of fungal endophytes on interspecific and intraspecific competition in the grasses Festuca arundinacea and Lolium perenne. J. Appl. Ecol. 1991, 28, 194–204. [Google Scholar] [CrossRef]
- Feistel, R.; Weinreben, S.; Wolf, H.; Seitz, S.; Spitzer, P.; Adel, B.; Nausch, G.; Schneider, B.; Wright, D.G. Density and absolute salinity of the Baltic Sea 2006–2009. Ocean Sci. 2010, 6, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.C.; Jackson, M.A.; Johnson-Cicalese, J.M. A Rapid staining method for detection of endophytic fungi in turf and forage grasses. Phytopathology 1988, 78, 237–239. [Google Scholar] [CrossRef]
- R Core Team R: A Language and environment for statistical computing. 2020. Available online: https://www.R-project.org/ (accessed on 1 September 2021).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.3.4. Available online: https://CRAN.R-Project.Org/Package=emmeans2019 (accessed on 1 September 2021).
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.-Q.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 20, 70. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, J.; Pour-Aboughadareh, A.; Fabriki Ourang, S.; Khalili, P.; Poczai, P. Unraveling salinity stress responses in ancestral and neglected wheat species at early growth stage: A baseline for utilization in future wheat improvement programs. Physiol. Mol. Biol. Plants 2020, 26, 537–549. [Google Scholar] [CrossRef]
- Saikkonen, K.; Ion, D.; Gyllenberg, M. The persistence of vertically transmitted fungi in grass metapopulations. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faeth, S.H.; Helander, M.L.; Saikkonen, K.T. Asexual Neotyphodium endophytes in a native grass reduce competitive abilities. Ecol. Lett. 2004, 7, 304–313. [Google Scholar] [CrossRef]
- Helander, M.; Phillips, T.; Faeth, S.H.; Bush, L.P.; McCulley, R.; Saloniemi, I.; Saikkonen, K. Alkaloid quantities in endophyte-infected tall fescue are affected by the plant-fungus combination and environment. J. Chem. Ecol. 2016, 42, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Schardl, C.L.; Florea, S.; Pan, J.; Nagabhyru, P.; Bec, S.; Calie, P.J. The Epichloae: Alkaloid diversity and roles in symbiosis with grasses. Curr. Opin. Plant Biol. 2013, 16, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Li, X.; Saikkonen, K.; Li, C.; Nan, Z. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Casler, M.D.; van Santen, E. Fungal endophyte removal does not reduce cold tolerance of tall fescue. Crop. Sci. 2008, 48, 2033–2039. [Google Scholar] [CrossRef]

| Total Biomass (log) | Root:Shoot Ratio (log) | Chlorophyll Content | ||||
|---|---|---|---|---|---|---|
| Explanatory Variables | Fdf,ddf | p | Fdf,ddf | p | Fdf,ddf | p |
| Endophyte (E) | 5.351,28 | 0.028 | 11.451,28 | 0.002 | 35.471,28 | <0.001 |
| Salt (S) | 1115.931,322 | <0.001 | 1472.581,322 | <0.001 | 582.121,322 | <0.001 |
| Competition (C) | 135.161,322 | <0.001 | 10.711,322 | 0.001 | 50.301,322 | <0.001 |
| E × S | 3.991,322 | 0.047 | 1.401,322 | 0.238 | 0.031,322 | 0.870 |
| E × C | 0.021,322 | 0.878 | 2.031,322 | 0.155 | 0.001,322 | 0.973 |
| S × C | 24.851,322 | <0.001 | 10.571,322 | 0.001 | 0.181,322 | 0.676 |
| E × S × C | 0.101,322 | 0.752 | 0.051,322 | 0.816 | 0.141,322 | 0.711 |
| Shoot Biomass 1st year (sqrt) | Height June 2nd year | Shoot Biomass 2nd year (log) | Survival | Flowering Probability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Explanatory Variables | Fdf,ddf | p | Fdf,ddf | p | Fdf,ddf | p | X2 | p | X2 | p |
| Endophyte (E) | 0.261,28 | 0.616 | 9.101,25 | 0.006 | 0.011,27 | 0.921 | 1.29 | 0.256 | 0.94 | 0.333 |
| Salt (S) | 5.441,82 | 0.022 | 1.211,69 | 0.274 | 5.031,70 | 0.028 | 10.89 | 0.001 | 0.62 | 0.431 |
| Competition (C) | 1.701,82 | 0.196 | 1.111,64 | 0.297 | 0.801,64 | 0.375 | 0.48 | 0.489 | 1.17 | 0.279 |
| E × S | 0.181,82 | 0.671 | 0.021,69 | 0.898 | 0.661,70 | 0.419 | 0.45 | 0.503 | 0.67 | 0.413 |
| E × C | 1.671,82 | 0.200 | 0.881,64 | 0.352 | 0.801,64 | 0.374 | 0.37 | 0.543 | 0.09 | 0.770 |
| S × C | 0.931,82 | 0.337 | 1.421,68 | 0.237 | 0.951,69 | 0.332 | 0.15 | 0.695 | 0.43 | 0.512 |
| E × S × C | 3.431,82 | 0.068 | 4.801,68 | 0.032 | 1.441,69 | 0.234 | 0.39 | 0.535 | 4.84 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalske, A.; Saikkonen, K.; Helander, M. Endophytic Fungus Negatively Affects Salt Tolerance of Tall Fescue. J. Fungi 2023, 9, 14. https://doi.org/10.3390/jof9010014
Kalske A, Saikkonen K, Helander M. Endophytic Fungus Negatively Affects Salt Tolerance of Tall Fescue. Journal of Fungi. 2023; 9(1):14. https://doi.org/10.3390/jof9010014
Chicago/Turabian StyleKalske, Aino, Kari Saikkonen, and Marjo Helander. 2023. "Endophytic Fungus Negatively Affects Salt Tolerance of Tall Fescue" Journal of Fungi 9, no. 1: 14. https://doi.org/10.3390/jof9010014







