50 Years of Cumulative Open-Source Data Confirm Stable and Robust Biodiversity Distribution Patterns for Macrofungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurrence Data of Macrofungal Species
2.2. Predictor Variables
2.3. Species Distribution Modelling
2.4. Calculation of Macrofungal Species Richness and Hotspots
3. Results
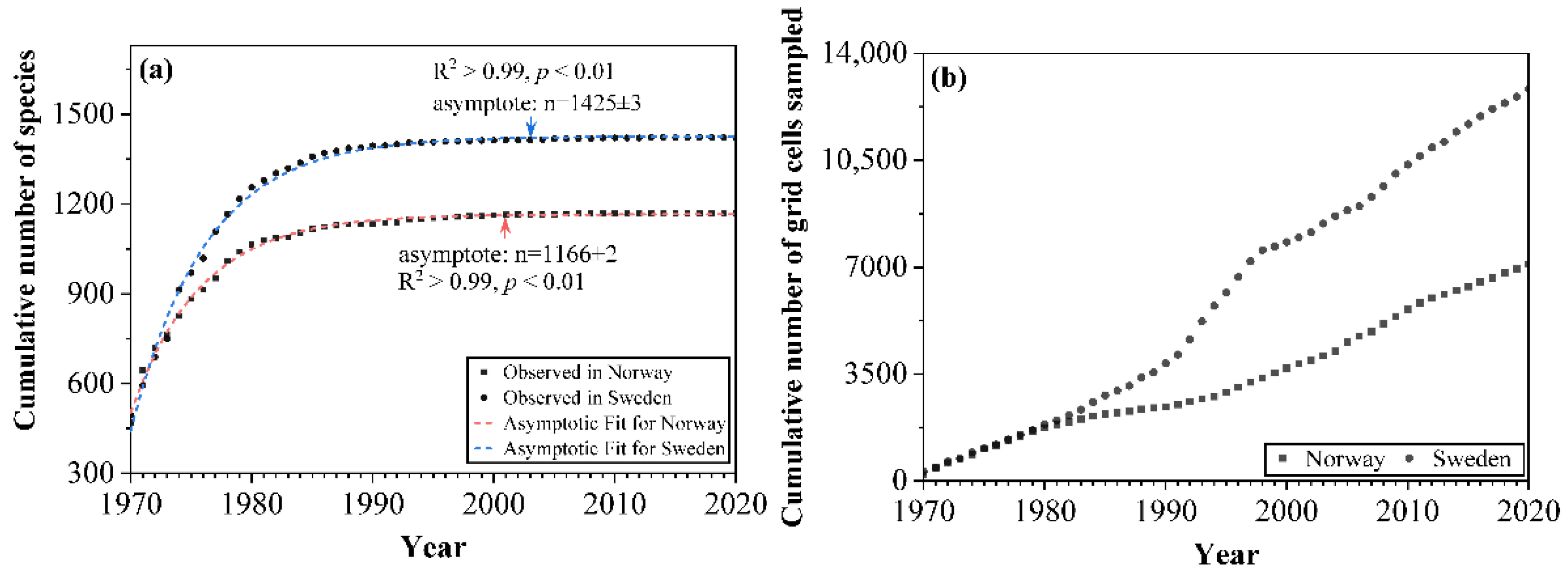
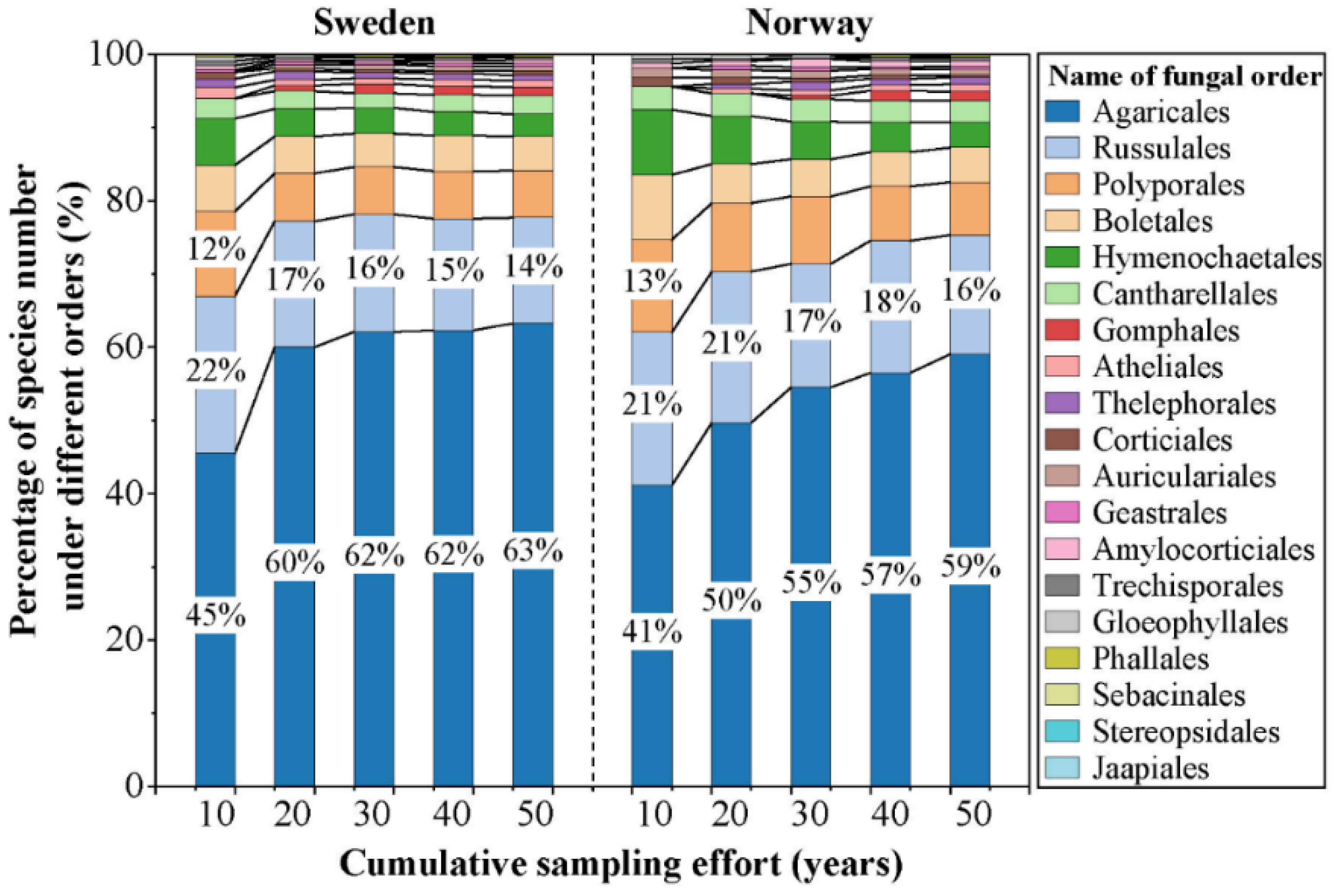
3.1. Accumulation of Macrofungal Species over Time
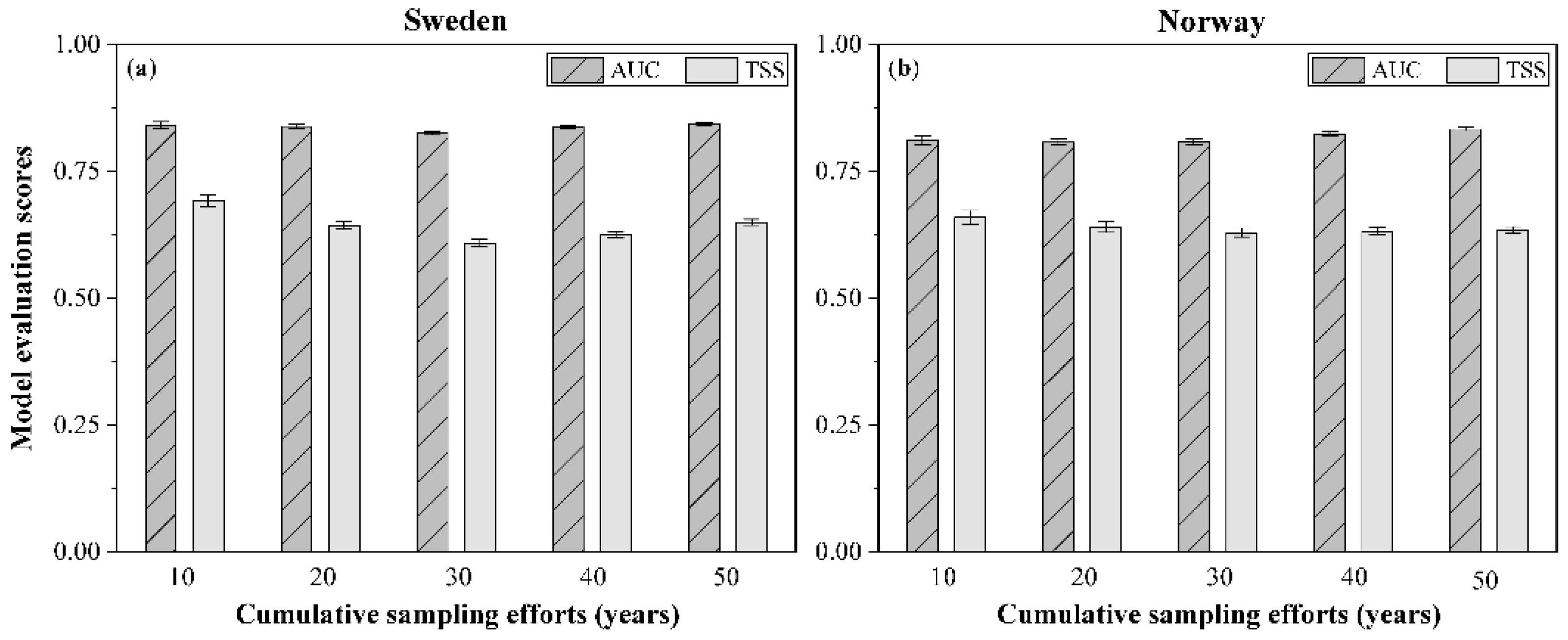
3.2. Model Performance
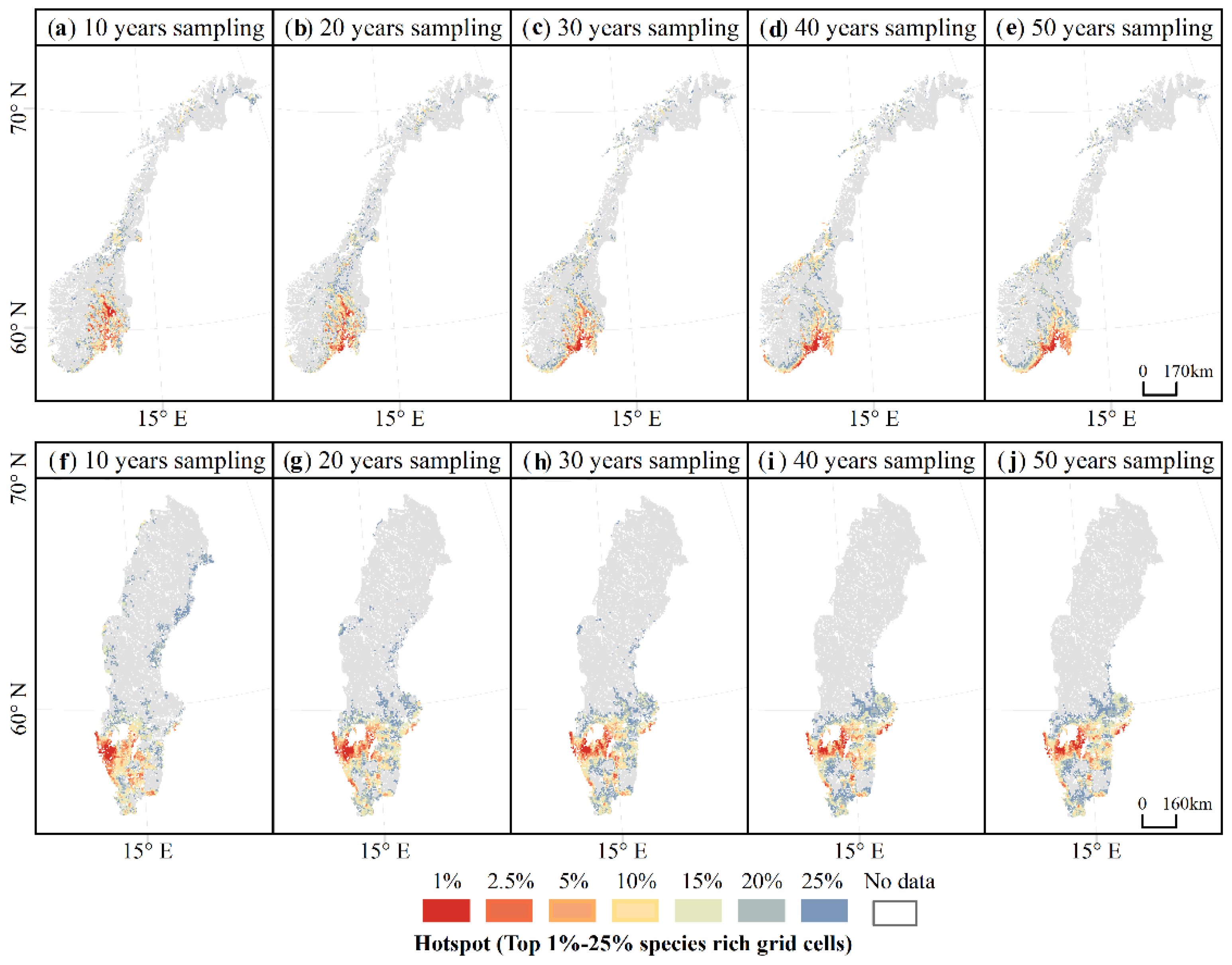
3.3. Predicted Macrofungal Species Richness and Diversity Hotspots
4. Discussion
4.1. The Importance of Long-Term Accumulation of Macrofungal Data
4.2. Uncertainty and Limitations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The Fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Functional and ecological consequences of saprotrophic fungus–grazer interactions. The ISME J. 2012, 6, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Lentendu, G.; Zinger, L.; Manel, S.; Coissac, E.; Choler, P.; Geremia, R.A.; Melodelima, C. Assessment of soil fungal diversity in different alpine tundra habitats by means of pyrosequencing. Fungal Divers. 2011, 49, 113–123. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal biodiversity: What do we know? What can we predict? Biodivers. Conserv. 2007, 16, 1–5. [Google Scholar] [CrossRef]
- Truong, C.; Mujic, A.B.; Healy, R.; Kuhar, F.; Furci, G.; Torres, D.; Niskanen, T.; Sandoval-Leiva, P.A.; Fernández, N.; Escobar, J.M. How to know the fungi: Combining field inventories and DNA-barcoding to document fungal diversity. N. Phytol. 2017, 214, 913–919. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Schmit, J.P.; Mueller, G.M.; Leacock, P.R.; Mata, J.L.; Huang, Y. Assessment of tree species richness as a surrogate for macrofungal species richness. Biol. Conserv. 2005, 121, 99–110. [Google Scholar] [CrossRef]
- Watkinson, S.C.; Boddy, L.; Money, N. The Fungi, 3rd ed.; Academic Press: London, UK, 2015. [Google Scholar]
- Heegaard, E.; Boddy, L.; Diez, J.; Halvorsen, R.; Kauserud, H.; Kuyper, T.; Bässler, C.; Büntgen, U.; Gange, A.; Krisai-Greilhuber, I. Fine-scale spatiotemporal dynamics of fungal fruiting: Prevalence, amplitude, range and continuity. Ecography 2017, 40, 947–959. [Google Scholar] [CrossRef]
- Richard, F.; Millot, S.; Gardes, M.; Selosse, M.A. Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex. N. Phytol. 2005, 166, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Straatsma, G.; François, A.; Simon, E. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef]
- Wollan, A.K.; Bakkestuen, V.; Kauserud, H.; Gulden, G.; Halvorsen, R. Modelling and predicting fungal distribution patterns using herbarium data. J. Biogeogr. 2008, 35, 2298–2310. [Google Scholar] [CrossRef]
- Lodge, D.J.; Ammirati, J.F.; O’Dell, T.E.; Mueller, G.M. Collecting and describing macrofungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 128–158. [Google Scholar]
- Kües, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000, 54, 141–152. [Google Scholar] [CrossRef]
- Lendzian, K.J.; Beck, A. Barrier properties of fungal fruit body skins, pileipelles, contribute to protection against water loss. Sci. Rep. 2021, 11, 8736. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: New York, NY, USA, 2011. [Google Scholar]
- McKnight, K.B.; Rohrer, J.R.; Ward, K.M.; McKnight, K.H. Peterson Field Guide to Mushrooms of North America; Mariner Books: Boston, MA, USA, 2021. [Google Scholar]
- Egli, S.; Ayer, F.; Chatelain, F. Die beschreibung der diversität von makromyzeten. Erfahrungen aus pilzökologischen langzeitstudien im pilzreservat la chanéaz, FR. Mycol. Helv. 1997, 9, 19–32. [Google Scholar]
- Straatsma, G.; Krisai-Greilhuber, I. Assemblage structure, species richness, abundance, and distribution of fungal fruit bodies in a seven year plot-based survey near Vienna. Mycol. Res. 2003, 107, 632–640. [Google Scholar] [CrossRef]
- Graham, C.H.; Ferrier, S.; Huettman, F.; Moritz, C.; Peterson, A.T. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef]
- Deshwal, A.; Panwar, P.; Neal, J.C.; Young, M.A. Using long-term citizen science data to understand distribution and habitat use of an irruptive species. Ecol. Inform. 2021, 64, 101377. [Google Scholar] [CrossRef]
- Newbold, T. Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Prog. Phys. Geogr. 2010, 34, 3–22. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Copas, K.; Bonde, A.M.; López, B.C.; Danielsen, F.; Legind, J.K.; Masinde, S.; Miller-Rushing, A.J.; Newman, G.; et al. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2017, 213, 280–294. [Google Scholar] [CrossRef]
- Engemann, K.; Enquist, B.J.; Sandel, B.; Boyle, B.; Jørgensen, P.M.; Morueta-Holme, N.; Peet, R.K.; Violle, C.; Svenning, J.C. Limited sampling hampers “big data” estimation of species richness in a tropical biodiversity hotspot. Ecol. Evol. 2015, 5, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, H.H.; Scheffrahn, R.H.; Basille, M.; Boone, M. Evaluating the data quality of iNaturalist termite records. PLoS ONE 2020, 15, e0226534. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, S.; Buermann, W.; Ter Steege, H.; Mori, S.; Smith, T.B. Modeling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sens. Environ. 2008, 112, 2000–2017. [Google Scholar] [CrossRef]
- Soroye, P.; Ahmed, N.; Kerr, J.T. Opportunistic citizen science data transform understanding of species distributions, phenology, and diversity gradients for global change research. Global Change Biol. 2018, 24, 5281–5291. [Google Scholar] [CrossRef]
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating species diversity and distribution in the era of big data: To what extent can we trust public databases? Global Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ortega, M.; Rodriguez, P.; Córdoba-Aguilar, A. Geographical, temporal and taxonomic biases in insect GBIF data on biodiversity and extinction. Ecol. Entomol. 2021, 46, 718–728. [Google Scholar] [CrossRef]
- Dennis, R.; Thomas, C. Bias in butterfly distribution maps: The influence of hot spots and recorder’s home range. J. Insect Conserv. 2000, 4, 73–77. [Google Scholar] [CrossRef]
- Kadmon, R.; Farber, O.; Danin, A. Effect of roadside bias on the accuracy of predictive maps produced by bioclimatic models. Ecol. Appl. 2004, 14, 401–413. [Google Scholar] [CrossRef]
- Hortal, J.; Lobo, J.M.; Jiménez-Valverde, A. Limitations of biodiversity databases: Case study on seed-plant diversity in Tenerife, Canary Islands. Conserv. Biol. 2007, 21, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Hortal, J.; Jiménez-Valverde, A.; Gómez, J.F.; Lobo, J.M.; Baselga, A. Historical bias in biodiversity inventories affects the observed environmental niche of the species. Oikos 2008, 117, 847–858. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Pyke, G.H.; Ehrlich, P.R. Biological collections and ecological/environmental research: A review, some observations and a look to the future. Biol. Rev. 2010, 85, 247–266. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef]
- Mayani-Parás, F.; Botello, F.; Castañeda, S.; Munguía-Carrara, M.; Sánchez-Cordero, V. Cumulative habitat loss increases conservation threats on endemic species of terrestrial vertebrates in Mexico. Biol. Conserv. 2021, 253, 108864. [Google Scholar] [CrossRef]
- Cam, E.; Nichols, J.D.; Sauer, J.R.; Hines, J.E. On the estimation of species richness based on the accumulation of previously unrecorded species. Ecography 2002, 25, 102–108. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1994, 345, 101–118. [Google Scholar]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large-and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Andrew, C.; Büntgen, U.; Egli, S.; Senn-Irlet, B.; Grytnes, J.A.; Heilmann-Clausen, J.; Boddy, L.; Bässler, C.; Gange, A.C.; Heegaard, E. Open-source data reveal how collections-based fungal diversity is sensitive to global change. Appl. Plant Sci. 2019, 7, e01227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, C.; Halvorsen, R.; Heegaard, E.; Kuyper, T.W.; Heilmann-Clausen, J.; Krisai-Greilhuber, I.; Bässler, C.; Egli, S.; Gange, A.C.; Høiland, K. Continental-scale macrofungal assemblage patterns correlate with climate, soil carbon and nitrogen deposition. J. Biogeogr. 2018, 45, 1942–1953. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Bruun, H.H.; Ejrnæs, R.; Frøslev, T.G.; Læssøe, T.; Petersen, J.H. How citizen science boosted primary knowledge on fungal biodiversity in Denmark. Biol. Conserv. 2019, 237, 366–372. [Google Scholar] [CrossRef]
- Krah, F.-S.; Büntgen, U.; Schaefer, H.; Müller, J.; Andrew, C.; Boddy, L.; Diez, J.; Egli, S.; Freckleton, R.; Gange, A.C. European mushroom assemblages are darker in cold climates. Nat. Commun. 2019, 10, 2890. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T.; Skidmore, A.; Heurich, M.; Bässler, C. The critical role of tree species and human disturbance in determining the macrofungal diversity in Europe. Global Ecol. Biogeogr. 2021, 30, 2084–2100. [Google Scholar] [CrossRef]
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe; ECCF: Strasbourg, France, 2007. [Google Scholar]
- Sánchez-García, M.; Ryberg, M.; Khan, F.K.; Varga, T.; Nagy, L.G.; Hibbett, D.S. Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 32528–32534. [Google Scholar] [CrossRef]
- Andrew, C.; Heegaard, E.; Høiland, K.; Senn-Irlet, B.; Kuyper, T.W.; Krisai-Greilhuber, I.; Kirk, P.M.; Heilmann-Clausen, J.; Gange, A.C.; Egli, S. Explaining European fungal fruiting phenology with climate variability. Ecology 2018, 99, 1306–1315. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.; Li, J.; Peterson, A.T.; Graham, C.; Guisan, A.; Group, N.P.S.D.W. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Stevens, W. Asymptotic regression. Biometrics 1951, 1, 247–267. [Google Scholar] [CrossRef]
- Willis, K.J.E. State of the World’s Fungi 2018; Royal Botanic Gardens: Kew, Australia, 2018. [Google Scholar]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate variation effects on fungal fruiting. Fungal Ecol. 2014, 10, 20–33. [Google Scholar] [CrossRef]
- Van Der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N. Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. US Geol. Surv. Data Ser. 2012, 691, 4–9. [Google Scholar]
- Brus, D.; Hengeveld, G.; Walvoort, D.; Goedhart, P.; Heidema, A.; Nabuurs, G.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2012, 131, 145–157. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer: New York, NY, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.P.; Gallardo, B.; Terblanche, J.S. A global assessment of climatic niche shifts and human influence in insect invasions. Global Ecol. Biogeogr. 2017, 26, 679–689. [Google Scholar] [CrossRef]
- Tanaka, K.R.; Torre, M.P.; Saba, V.S.; Stock, C.A.; Chen, Y. An ensemble high-resolution projection of changes in the future habitat of American lobster and sea scallop in the Northeast US continental shelf. Divers. Distrib. 2020, 26, 987–1001. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Processing 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Jones, E.L.; Rendell, L.; Pirotta, E.; Long, J.A. Novel application of a quantitative spatial comparison tool to species distribution data. Ecol. Indic. 2016, 70, 67–76. [Google Scholar] [CrossRef]
- Vallejos, R.; Osorio, F.; Bevilacqua, M. Spatial Relationships Between Two Georeferenced Variables: With Application in R; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.-S.; Rasmussen, P.C.; Ridgely, R.S. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Guralnick, R.; Van Cleve, J. Strengths and weaknesses of museum and national survey data sets for predicting regional species richness: Comparative and combined approaches. Divers. Distrib. 2005, 11, 349–359. [Google Scholar] [CrossRef]
- Kelling, S.; Fink, D.; La Sorte, F.A.; Johnston, A.; Bruns, N.E.; Hochachka, W.M. Taking a ‘Big Data’approach to data quality in a citizen science project. Ambio 2015, 44, 601–611. [Google Scholar] [CrossRef]
- Rydin, H.; Diekmann, M.; Hallingbäck, T. Biological Characteristics, Habitat Associations, and Distribution of Macrofungi in Sweden: Características Biológicas, Asociaciones de Hábitat y Distribución de Macrohongos en Suecia. Conserv. Biol. 1997, 11, 628–640. [Google Scholar] [CrossRef]
- Hagen, D.; Svavarsdottir, K.; Nilsson, C.; Tolvanen, A.K.; Raulund-Rasmussen, K.; Aradòttir, À.L.; Fosaa, A.M.; Halldorsson, G. Ecological and social dimensions of ecosystem restoration in the Nordic countries. Ecol. Soc. 2013, 18, 34. [Google Scholar] [CrossRef]
- Nilsson, S.G.; Hedin, J.; Niklasson, M. Biodiversity and its assessment in boreal and nemoral forests. Scand. J. For. Res. 2001, 16, 10–26. [Google Scholar] [CrossRef]
- Troia, M.J.; McManamay, R.A. Filling in the GAPS: Evaluating completeness and coverage of open-access biodiversity databases in the United States. Ecol. Evol. 2016, 6, 4654–4669. [Google Scholar] [CrossRef]
- Fleming, A.; Howden, S. Ambiguity: A new way of thinking about responses to climate change. Sci. Total Environ. 2016, 571, 1271–1274. [Google Scholar] [CrossRef]
- Oldfield, F.; Steffen, W. Anthropogenic climate change and the nature of Earth System science. Anthr. Rev. 2014, 1, 70–75. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA–An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Buee, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. N. Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.-M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; Whittaker, R.J.; Beltrame, C. Beyond scarcity: Citizen science programmes as useful tools for conservation biogeography. Divers. Distrib. 2010, 16, 354–362. [Google Scholar] [CrossRef]



| 10 Years Sampling | 20 Years Sampling | 30 Years Sampling | 40 Years Sampling | 50 Years Sampling | |||
|---|---|---|---|---|---|---|---|
| Number of species | Sweden | original macrofungi | 1715 | 2243 | 2538 | 2783 | 2999 |
| macrofungi used in our model | 330 | 775 | 1053 | 1243 | 1422 | ||
| Norway | original macrofungi | 1717 | 2153 | 2420 | 2655 | 2901 | |
| macrofungi used in our model | 158 | 320 | 587 | 927 | 1168 | ||
| Number of grid cells | Sweden | original macrofungi | 1877 | 3900 | 7860 | 10,388 | 12,851 |
| macrofungi used in our model | 1581 | 3678 | 7742 | 10,324 | 12,830 | ||
| Norway | original macrofungi | 1800 | 2504 | 3774 | 5703 | 7167 | |
| macrofungi used in our model | 1296 | 2036 | 3515 | 5559 | 7100 | ||
| Variables | Description | Unit |
|---|---|---|
| Bio01 | Annual mean temperature | °C |
| Bio04 | Temperature seasonality | % |
| Bio07 | Temperature Annual Range | °C |
| Bio12 | Annual precipitation | mm |
| Bio15 | Precipitation seasonality | % |
| Dominantree | Dominant tree species | |
| Richness | Tree species richness | |
| Simpson’s Index | Diversity index of tree species | |
| pH | Soil pH in water | |
| SOC | Soil organic carbon content | g/kg |
| BD | Bulk density of the fine earth fraction | kg/dm3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, T.; Skidmore, A.; Heurich, M.; Bässler, C. 50 Years of Cumulative Open-Source Data Confirm Stable and Robust Biodiversity Distribution Patterns for Macrofungi. J. Fungi 2022, 8, 981. https://doi.org/10.3390/jof8090981
Yu H, Wang T, Skidmore A, Heurich M, Bässler C. 50 Years of Cumulative Open-Source Data Confirm Stable and Robust Biodiversity Distribution Patterns for Macrofungi. Journal of Fungi. 2022; 8(9):981. https://doi.org/10.3390/jof8090981
Chicago/Turabian StyleYu, Haili, Tiejun Wang, Andrew Skidmore, Marco Heurich, and Claus Bässler. 2022. "50 Years of Cumulative Open-Source Data Confirm Stable and Robust Biodiversity Distribution Patterns for Macrofungi" Journal of Fungi 8, no. 9: 981. https://doi.org/10.3390/jof8090981







