Exploring Biocontrol of Unwanted Fungi by Autochthonous Debaryomyces hansenii Strains Isolated from Dry Meat Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Primary Screening: Pathogenic Fungi Colonization Prevention Assay with Biofilms from D. hansenii Strains
2.3. Biotyping
2.4. Secondary Screening: Streak Inhibition Assay
2.5. Radial Inhibition Assay on Solid Media
2.6. Volatile Compounds Inhibition Assay
2.7. Data Quantification and Statistical Analysis
3. Results
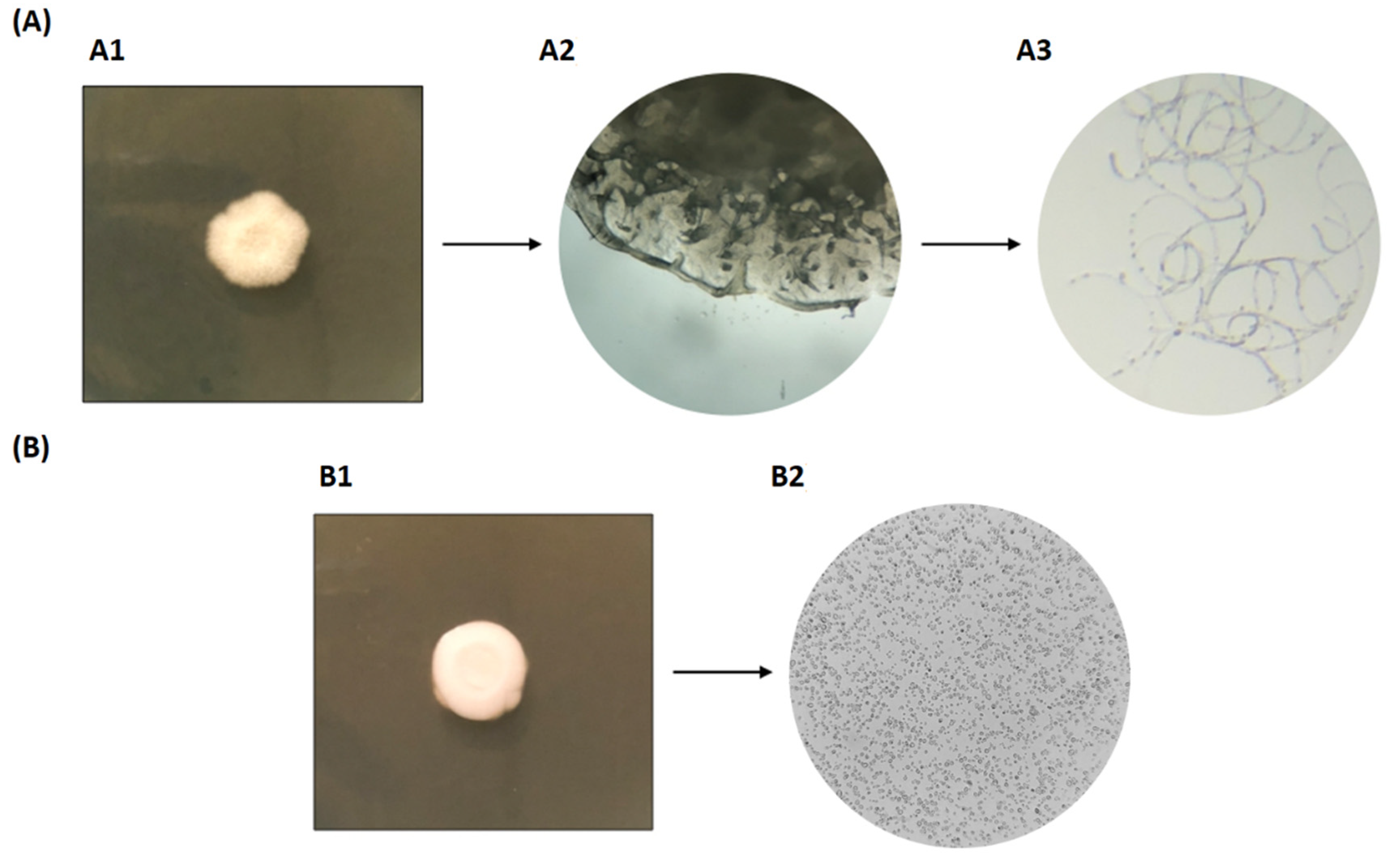
3.1. Primary Screening
3.2. Biotyping
3.3. Secondary Screening: Streak Inhibition Assay
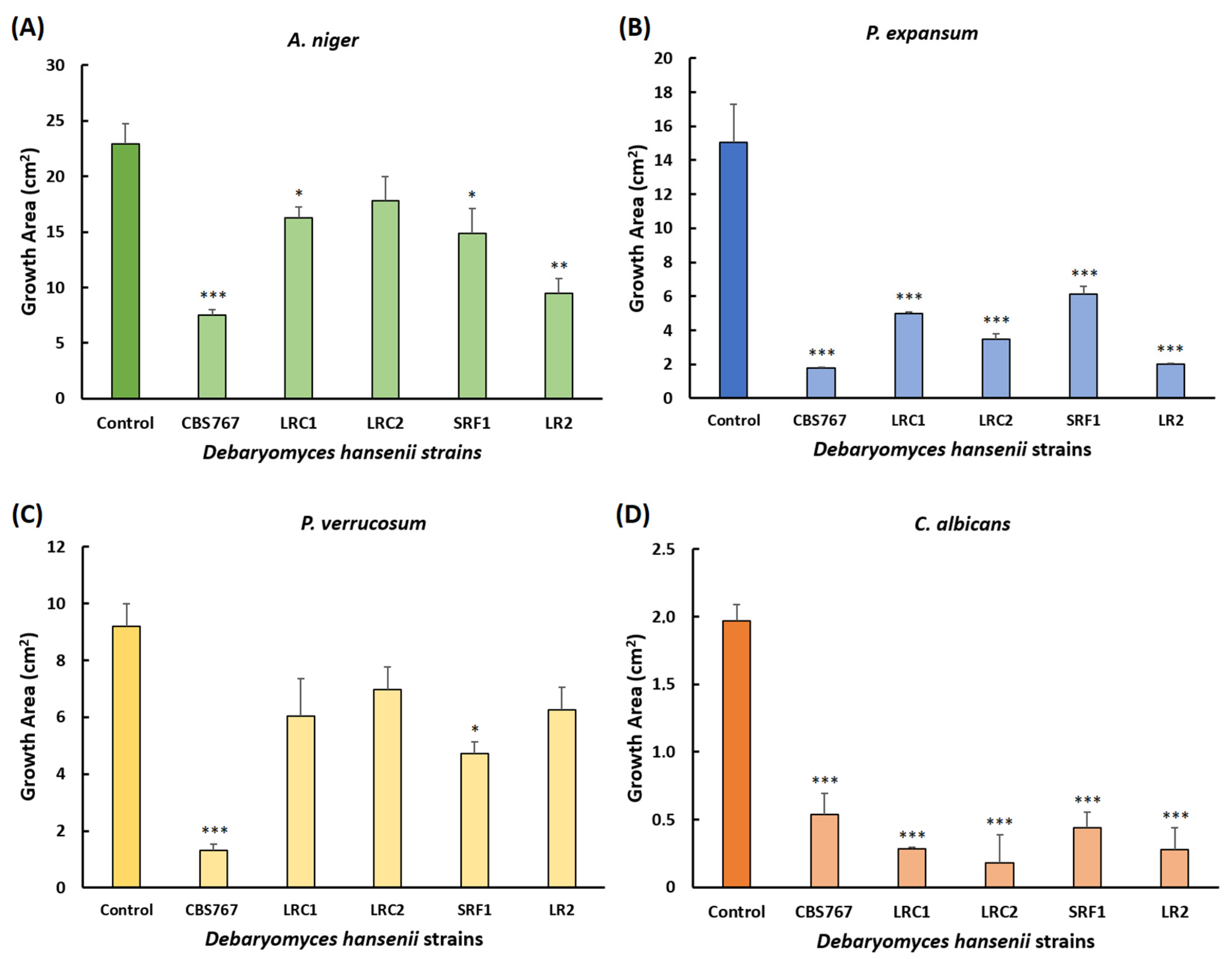
3.4. Radial Inhibition Assay
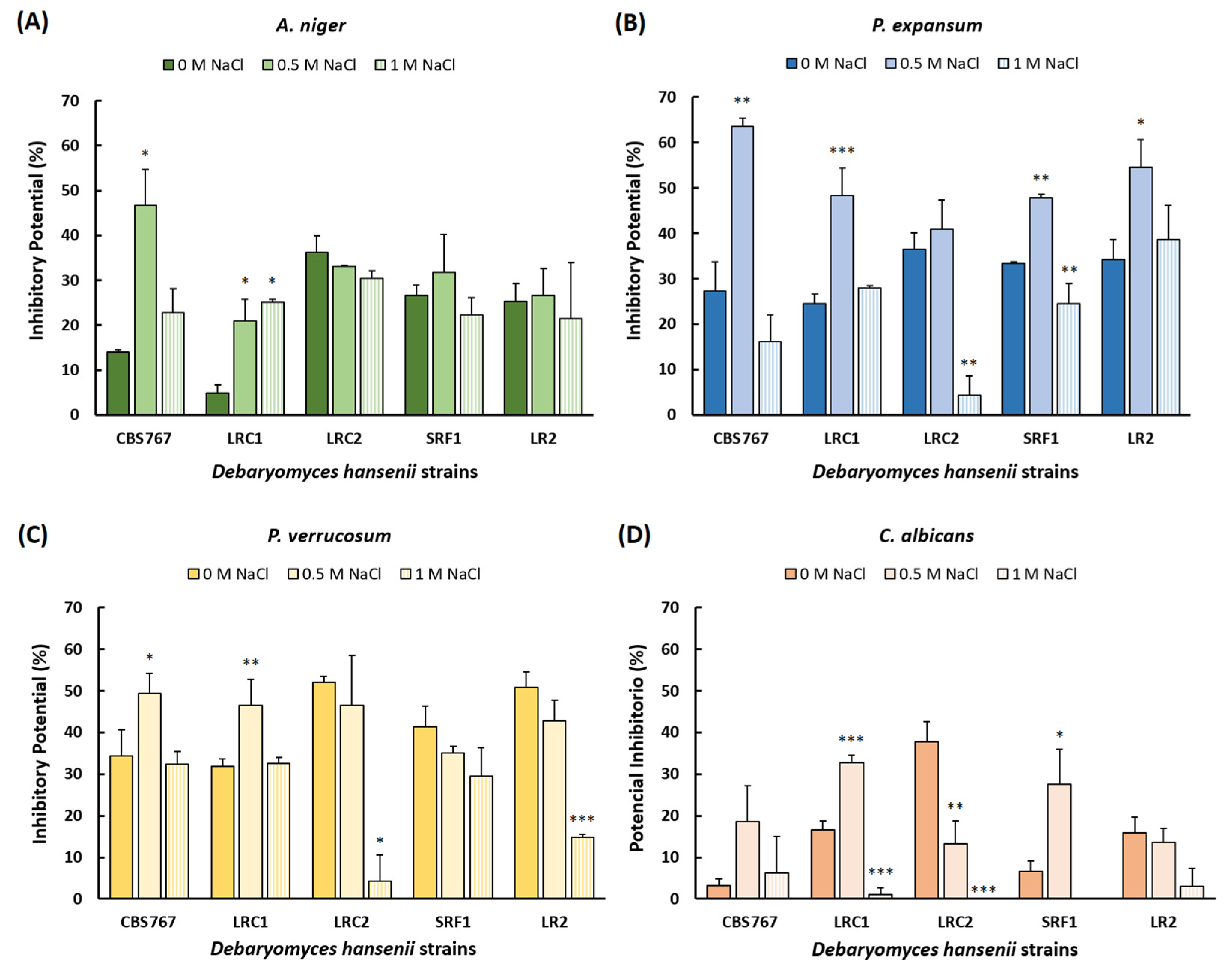
3.5. Volatile Compounds Inhibition Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-García, R.; Searle, S.S. Preservatives: Food use. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 505–509. [Google Scholar] [CrossRef]
- Peromingo, B.; Núñez, F.; Rodríguez, A.; Alía, A.; Andrade, M. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018, 268, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Micotoxinas. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/micotoxinas.htm (accessed on 26 June 2022).
- Leyva-Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation-A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, M.; Majcher, M.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Sharma, P.; Meena, N.; Aggarwal, M.; Mondal, A.K. Debaryomyces hansenii, a highly osmo-tolerant and halo-tolerant yeast, maintains activated Dhog1p in the cytoplasm during its growth under severe osmotic stress. Curr. Genet. 2005, 48, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.; Blomberg, A.; Nilsson, A. Glycerol metabolism and osmoregulation in the salt-tolerant yeast Debaryomyces hansenii. J. Bacteriol. 1985, 162, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Prista, C.; Almagro, A.; Loureiro-Dias, M.C.; Ramos, J. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl. Environ. Microbiol. 1997, 63, 4005–4009. [Google Scholar] [CrossRef]
- Prista, C.; Loureiro-Dias, M.C.; Montiel, V.; Garcia, R.; Ramos, J. Mechanisms underlying the halotolerant way of Debaryomyces hansenii. FEMS Yeast Res. 2005, 5, 693–701. [Google Scholar] [CrossRef]
- Ramos, J.; Melero, Y.; Ramos-Moreno, L.; Michán, C.; Cabezas, L. Debaryomyces hansenii Strains from Valle De Los Pedroches Iberian Dry Meat Products: Isolation, Identification, Characterization, and Selection for Starter Cultures. J. Microbiol. Biotechnol. 2017, 27, 1576–1585. [Google Scholar] [CrossRef]
- Murgia, M.; Marongiu, A.; Aponte, M.; Blaiotta, G.; Deiana, P.; Mangia, N. Impact of a selected Debaryomyces hansenii strain’s inoculation on the quality of Sardinian fermented sausages. Food Res. Int. 2019, 121, 144–150. [Google Scholar] [CrossRef]
- Banjara, N.; Nickerson, K.; Suhr, M.; Hallen-Adams, H. Killer toxin from several food-derived Debaryomyces hansenii strains effective against pathogenic Candida yeasts. Int. J. Food Microbiol. 2016, 222, 23–29. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Peris, D.; Belloch, C.; Flores, M. Debaryomyces hansenii Metabolism of Sulfur Amino Acids As Precursors of Volatile Sulfur Compounds of Interest in Meat Products. J. Agric. Food Chem. 2019, 67, 9335–9343. [Google Scholar] [CrossRef] [PubMed]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Muñoz, C.J.; Combina, M.; Toro, M.E.; Castellanos de Figueroa, L.I.; Vázquez, F. Biocontrol of Botrytis cinerea in table grapes by non-pathogenic indigenous Saccharomyces cerevisiae yeasts isolated from viticultural environments in Argentina. Postharvest Biol. Technol. 2012, 64, 40–48. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Technol. 2014, 88, 72–78. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Update of the list of QPS-re- commended biological agents intentionally added to food or feed as notified to EFSA 4: Suitability of taxonomic units notified to EFSA until March 2016. EFSA J. 2016, 14, 5965. [Google Scholar] [CrossRef]
- Virgili, R.; Simoncini, N.; Toscani, T.; Camardo Leggieri, M.; Formenti, S.; Battilani, P. Biocontrol of Penicillium nordicum growth and Ochratoxin A production by Native Yeasts of Dry Cured Ham. Toxins 2012, 4, 68–82. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry- cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.J.; Villalobos, M.C.; Martín, A.; Córdoba, M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef]
- Van Veen, S.Q.; Claas, E.C.; Kuijper, E.J. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010, 48, 900–907. [Google Scholar] [CrossRef]
- Prista, C.; Michán, C.; Miranda, I.; Ramos, J. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tsao, M. Biocontrol of dairy moulds by antagonistic dairy yeast Debaryomyces hansenii in yoghurt and cheese at elevated temperatures. Food Control 2009, 20, 852–855. [Google Scholar] [CrossRef]
- Navarrete, C.; Frost, A.T.; Ramos-Moreno, L.; Krum, M.R.; Martínez, J.L. A physiological characterization in controlled bioreactors reveals a novel survival strategy for Debaryomyces hansenii at high salinity. Yeast 2021, 38, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, N.; Pinna, A.; Toscani, T.; Virgili, R. Effect of added autochthonous yeasts on the volatile compounds of dry-cured hams. Int. J. Food Microbiol. 2015, 212, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaysi, S.; Al-Haideri, H.; Thabit, Z.A.; Al-Kubaisy, W.; Ibrahim, J. Production, Characterization, and Antimicrobial Activity of Mycocin Produced by Debaryomyces hansenii DSMZ70238. Int. J. Microbiol. 2017, 2017, 2605382. [Google Scholar] [CrossRef]
- García-Bramasco, C.A.; Blancas-Benitez, F.J.; Montaño-Leyva, B.; Medrano-Castellón, L.M.; Gutierrez-Martinez, P.; González-Estrada, R.R. Influence of Marine Yeast Debaryomyces hansenii on Antifungal and Physicochemical Properties of Chitosan-Based Films. J. Fungi 2022, 8, 369. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, L.; Johansen, P.G.; Petersen, M.A.; Arneborg, N.; Jespersen, L. Debaryomyces hansenii Strains Isolated From Danish Cheese Brines Act as Biocontrol Agents to Inhibit Germination and Growth of Contaminating Molds. Front. Microbiol. 2021, 12, 662785. [Google Scholar] [CrossRef]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef]
| Isolation Origin | Debaryomyces hansenii Strains | Aspergillus niger | Penicillium expansum | Penicillium verrucosum | Candida albicans |
|---|---|---|---|---|---|
| Laboratory control | CBS767 | + + + | + + + | + + + | + + + |
| “Chorizo Ibérico” | CB1 | + + + | + + + | + + + | + + + |
| CB2 | + | + + + | + + + | + + + | |
| CRC1 | + | + + + | + + + | + + + | |
| CRC2 | + | + + + | + + + | + + + | |
| CRF1 | + + | + + + | + + + | + + + | |
| CRF2 | + + | + + + | + + + | + + + | |
| CRF3 | + | + | + | + | |
| “Lomo Ibérico” | LB1 | + + + | + + + | + + + | + + + |
| LB2 | + + + | + + + | + + + | + + + | |
| LB3 | + | + + + | + + + | + + + | |
| LR1 | + + + | + + + | + + + | + + + | |
| LR2 | + + + | + + + | + + + | + + + | |
| LRB1 | + + + | + + + | + + + | + + + | |
| LRB2 | + + + | + + + | + + + | + + + | |
| LRB3 | + + + | + + + | + + + | + + + | |
| LRB4 | + + + | + + + | + + + | + + + | |
| LRC1 | + + + | + + + | + + + | + + + | |
| LRC2 | + + + | + + + | + + + | + + + | |
| LRF1 | + + | + + + | + + + | + + + | |
| LRF2 | + + + | + + + | + | + | |
| “Salchichón Ibérico” | SRC1 | + + + | + + + | + + + | + + + |
| SRC2 | + + + | + + + | + + + | + + + | |
| SRF1 | + + + | + + + | + + + | + + + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Navarrete, H.; Ruiz-Pérez, F.; Ruiz-Castilla, F.J.; Ramos, J. Exploring Biocontrol of Unwanted Fungi by Autochthonous Debaryomyces hansenii Strains Isolated from Dry Meat Products. J. Fungi 2022, 8, 873. https://doi.org/10.3390/jof8080873
Chacón-Navarrete H, Ruiz-Pérez F, Ruiz-Castilla FJ, Ramos J. Exploring Biocontrol of Unwanted Fungi by Autochthonous Debaryomyces hansenii Strains Isolated from Dry Meat Products. Journal of Fungi. 2022; 8(8):873. https://doi.org/10.3390/jof8080873
Chicago/Turabian StyleChacón-Navarrete, Helena, Francisco Ruiz-Pérez, Francisco J. Ruiz-Castilla, and José Ramos. 2022. "Exploring Biocontrol of Unwanted Fungi by Autochthonous Debaryomyces hansenii Strains Isolated from Dry Meat Products" Journal of Fungi 8, no. 8: 873. https://doi.org/10.3390/jof8080873






