Stemphylium lycopersici Nep1-like Protein (NLP) Is a Key Virulence Factor in Tomato Gray Leaf Spot Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Fungal Strain Isolation and Growth Conditions
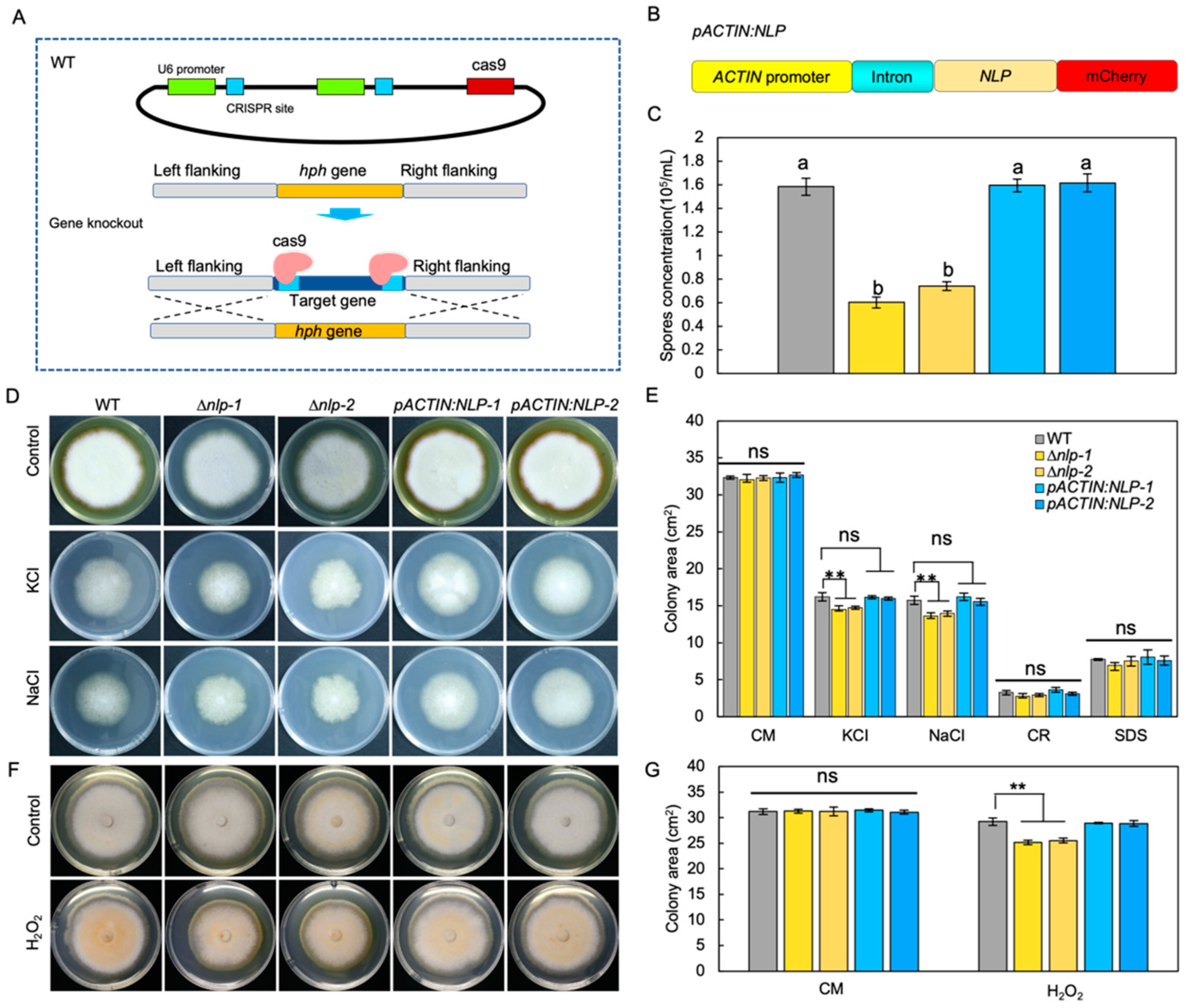
2.3. Gene Knockout and Overexpression in S. lycopersici
2.4. Genetic Transformation of S. lycopersici
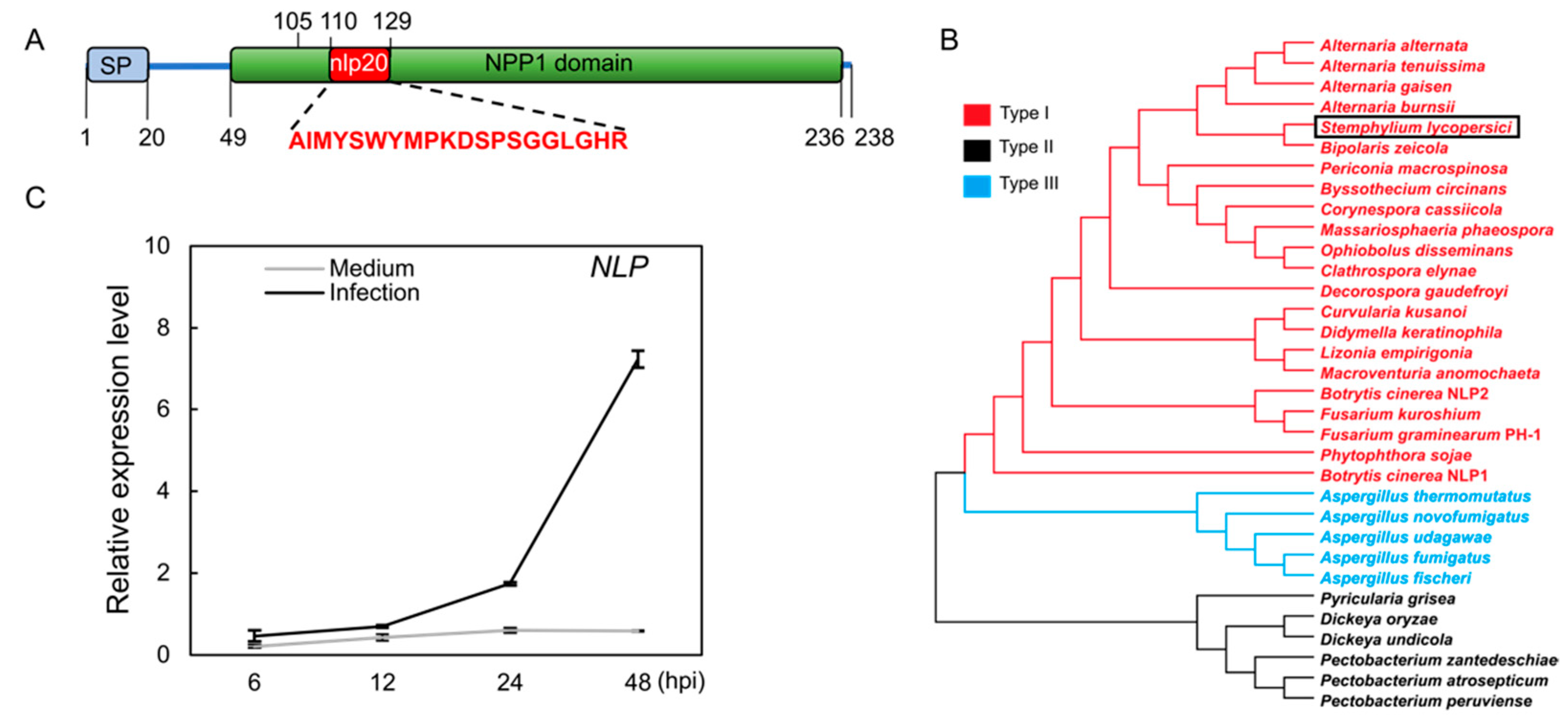
2.5. Bioinformatics Analysis and Phylogenetic Assay
2.6. Tomato Disease Assay
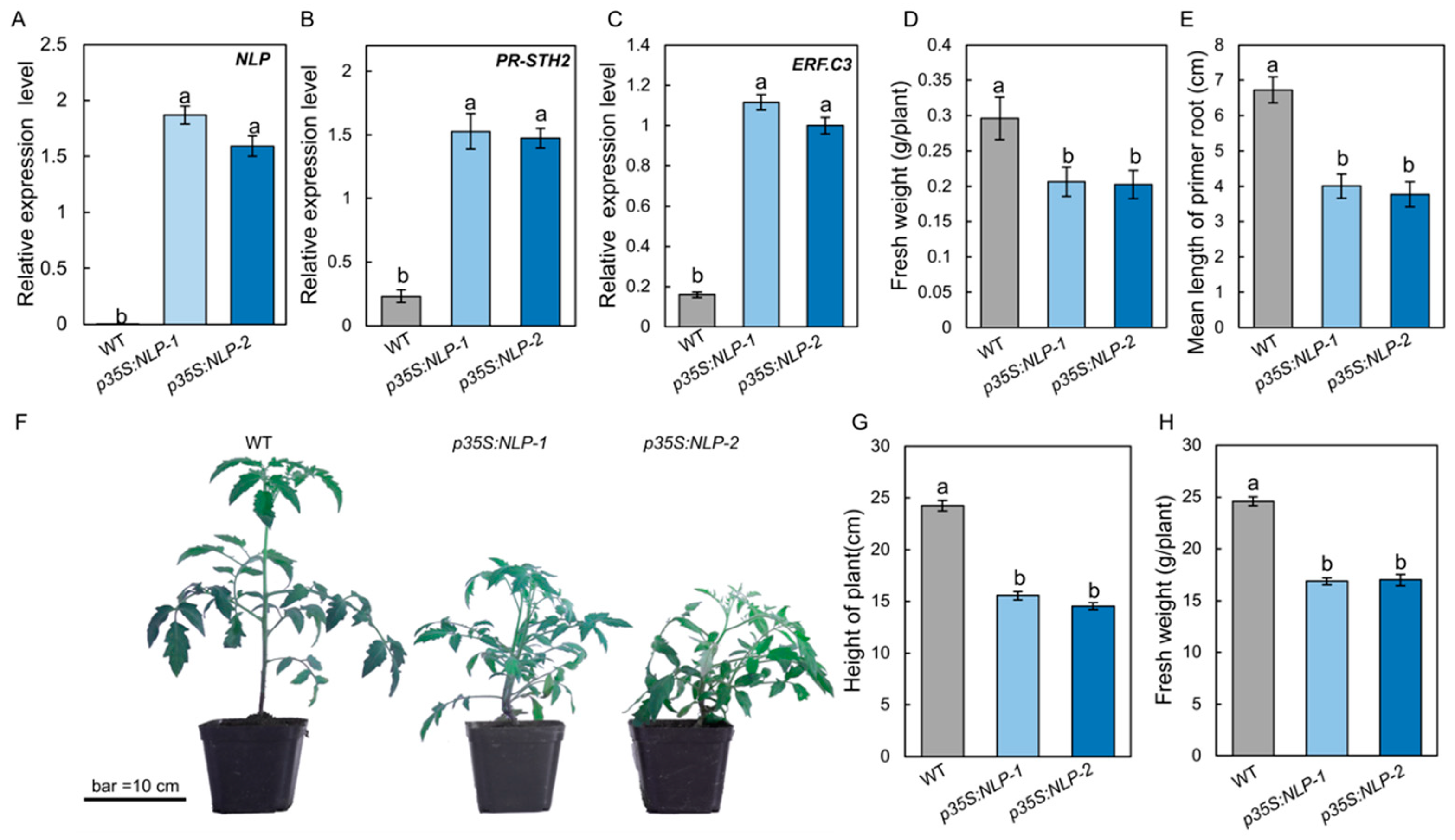
2.7. DNA Constructs and Plant Transformation
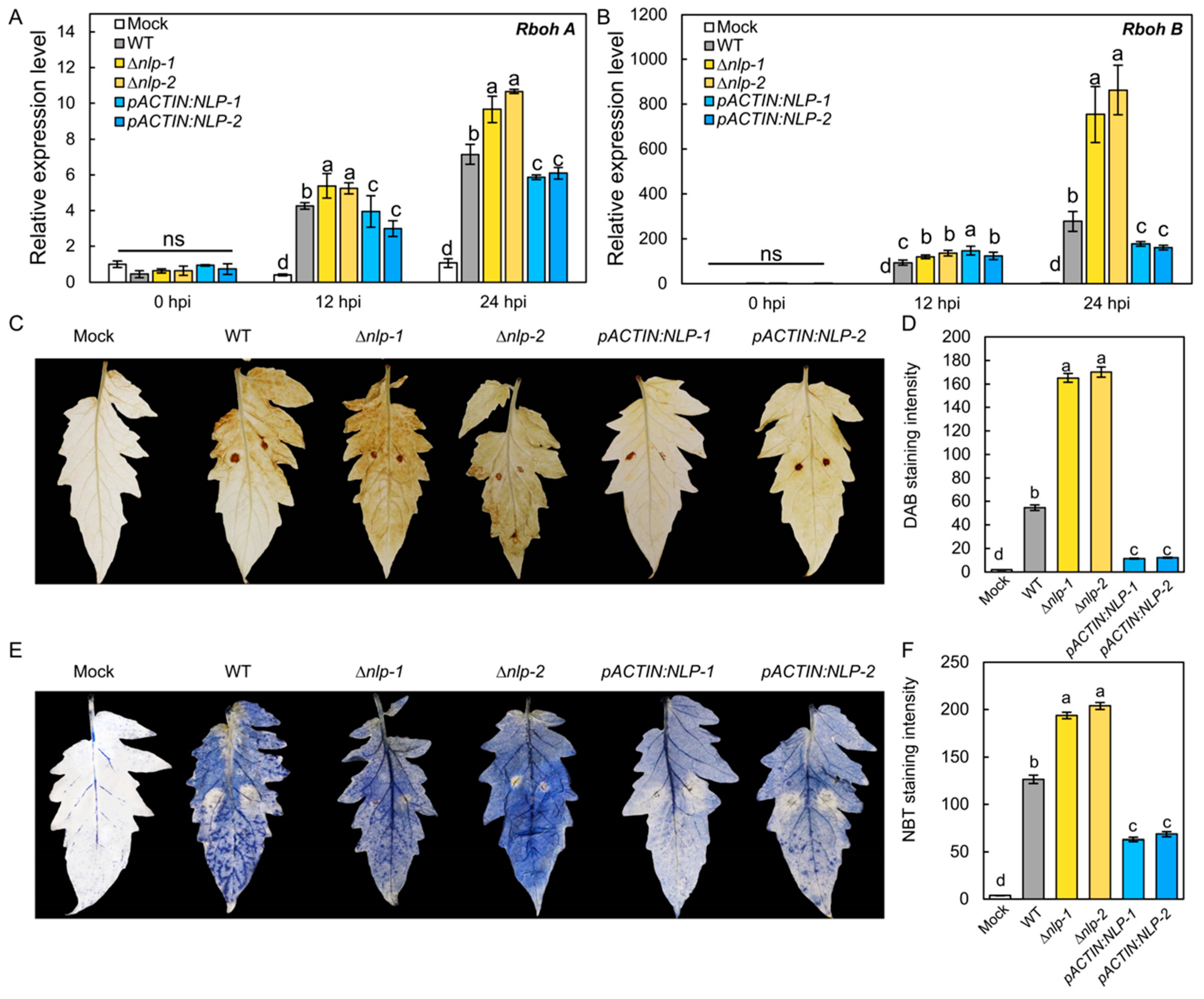
2.8. ROS Staining
2.9. RNA Extraction and RT-qPCR
2.10. Quantification and Statistical Analysis
3. Results
3.1. A Stemphylium lycopersici Type I Nep1-like Protein (NLP) Is Induced during Infection of Tomato
3.2. NLP Affects Conidiospore Production but Not Vegetative Growth in S. lycopersici
3.3. NLP Weakly Affects Osmotic and Oxidative Stress Adaptation in S. lycopersici
3.4. NLP Is Required for Full Virulence of S. lycopersici in Tomato
3.5. NLP Suppresses S. lycopersici Infection-Induced ROS Production in Tomato Leaves
3.6. Expression of S. lycopersici NLP in Tomato Leads to Constitutive Plant Immune Responses and Reduced Growth
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasehi, A.; Kadir, J.-B.; Nasr-Esfahani, M.; Abed-Ashtiani, F.; Wong, M.-Y.; Rambe, S.-K.; Golkhandan, E. Analysis of genetic and virulence variability of Stemphylium lycopersici associated with leaf spot of vegetable crops. Eur. J. Plant Pathol. 2014, 140, 261–273. [Google Scholar] [CrossRef]
- Franco, M.E.E.; Troncozo, M.I.; López, S.M.Y.; Lucentini, G.; Medina, R.; Saparrat, M.C.N.; Ronco, L.B.; Balatti, P.A. A survey on tomato leaf grey spot in the two main production areas of Argentina led to the isolation of Stemphylium lycopersici representatives which were genetically diverse and differed in their virulence. Eur. J. Plant Pathol. 2017, 149, 983–1000. [Google Scholar] [CrossRef] [Green Version]
- Koike, S.T.; O’Neill, N.; Wolf, J.; Van Berkum, P.; Daugovish, O. Stemphylium Leaf Spot of Parsley in California Caused by Stemphylium vesicarium. Plant Dis. 2013, 97, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvez, L.; Gil-Serna, J.; Garcia, M.; Iglesias, C.; Palmero, D. Stemphylium leaf blight of garlic (Allium sativum) in Spain: Taxonomy and in vitro fungicide response. Plant Pathol. J. 2016, 32, 388–395. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhong, J.; Lu, X.; Pan, X.T.; Zhu, H.J.; Zhou, Q. First report of Stemphylium lycopersici and Stemphylium vesicarium causing leaf spot on lettuce (Lactuca sativa) in China. Plant Dis. 2019, 103, 2598–2957. [Google Scholar] [CrossRef]
- Behare, J.; Laterrot, H.; Sarfatti, M.; Zamir, D. Restriction fragment length polymorphism mapping of the Stemphylium resistance gene in tomato. Mol. Plant-Microbe Interact. 1991, 4, 489–492. [Google Scholar] [CrossRef]
- Blancard, D. Tomato Diseases, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Qutob, D.; Kemmerling, B.; Brunner, F.; Küfner, I.; Engelhardt, S.; Gust, A.A.; Luberacki, B.; Seitz, H.U.; Stahl, D.; Rauhut, T. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 2006, 18, 3721–3744. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Gao, S.; Xu, L.; Liu, X.; Dai, F. Prediction of pathogenesis-related secreted proteins from Stemphylium lycopersici. BMC Microbiol. 2018, 18, 191. [Google Scholar] [CrossRef]
- Trigos, A.; Mendoza, G.; Espinoza, C.; Salinas, A.; Fernandez, J.J.; Norte, M. The role of macrosporin in necrotic spots. Phytochem. Lett. 2011, 4, 122–125. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oome, S.; Raaymakers, T.M.; Cabral, A.; Samwel, S.; Böhm, H.; Albert, I.; Nürnberger, T.; Van Den Ackerveken, G. Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 16955–16960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Dong, X.; Li, J.; Wang, Y.; Cheng, Y.; Zhai, Y.; Li, X.; Wei, L.; Jing, M.; Dou, D. Type 2 Nep1-like proteins from the biocontrol oomycete Pythium oligandrum suppress Phytophthora capsici infection in solanaceous plants. J. Fungi 2021, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Seidl, M.F.; Van Den Ackerveken, G. Activity and phylogenetics of the broadly occurring family of microbial Nep1-like proteins. Annu. Rev. Phytopathol. 2019, 57, 367–386. [Google Scholar] [CrossRef]
- Ottmann, C.; Luberacki, B.; Küfner, I.; Koch, W.; Brunner, F.; Weyand, M.; Mattinen, L.; Pirhonen, M.; Anderluh, G.; Seitz, H.U.; et al. A common toxin fold mediates microbial attack and plant defense. Proc. Natl. Acad. Sci. USA 2009, 106, 10359–10364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, E.; Mise, K.; Takano, Y. RLP23 is required for Arabidopsis immunity against the grey mould pathogen Botrytis cinerea. Sci. Rep. 2020, 10, 13798. [Google Scholar] [CrossRef]
- Böhm, H.; Albert, I.; Oome, S.; Raaymakers, T.M.; Van Den Ackerveken, G.; Nürnberger, T. A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLoS Pathog. 2014, 10, e1004491. [Google Scholar] [CrossRef]
- Albert, I.; Böhm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, T.; Jiang, J.; Wang, S.; Wang, A.; Li, J.; Xu, X. Mapping and screening of the tomato Stemphylium lycopersici resistance gene, Sm, based on bulked segregant analysis in combination with genome resequencing. BMC Plant Biol. 2017, 17, 266. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Jiang, J.; Liu, M.; Liu, Z.; Tan, Y.; Zhao, T.; Zhang, H.; Chen, X.; Li, J.; et al. The Sm gene conferring resistance to gray leaf spot disease encodes an NBS-LRR (nucleotide-binding site-leucine-rich repeat) plant resistance protein in tomato. Theor. Appl. Genet. 2022. [Google Scholar] [CrossRef]
- Laluk, K.; Mengiste, T. Necrotroph attacks on plants: Wanton destruction or covert extortion? Arab. Book 2010, 8, e0136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, R.; Franco, M.E.E.; da Cruz Cabral, L.; Bahima, J.V.; Patriarca, A.; Balatti, P.A.; Saparrat, M.C.N. The secondary metabolites profile of Stemphylium lycopersici, the causal agent of tomato grey leaf spot, is complex and includes host and non-host specific toxins. Australas. Plant Pathol. 2020, 50, 105–115. [Google Scholar] [CrossRef]
- Barash, I.; Karr, A.L.; Strobel, G.A. Isolation and characterization of Stemphylin, a chromone glucoside from Stemphylium botryosum. Plant Physiol. 1975, 55, 646–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, M.E.; López, S.; Medina, R.; Saparrat, M.C.; Balatti, P. Draft Genome Sequence and Gene Annotation of Stemphylium lycopersici Strain CIDEFI-216. Genome Announc. 2015, 3, e01069-15. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Zhao, J.; Tzeng, D.T.W.; Liu, Y.; Deng, L.; Yang, T.; Zhai, Q.; Wu, F.; Huang, Z.; Zhou, M.; et al. MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef] [Green Version]
- Korabečná, M.; Liška, V.; Fajfrlík, K. PrimersITS1, ITS2 andITS4 detect the intraspecies variability in the internal transcribed spacers and 5.8S rRNA gene region in clinical isolates of fungi. Folia Microbiol. 2003, 48, 233–238. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, S.; Wang, Z.; Xu, H.; Liang, J.; Liu, H.; Wang, G.; Ding, M.; Wang, Q.; Gong, C.; et al. An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. Nat. Microbiol. 2019, 4, 1582–1591. [Google Scholar] [CrossRef]
- Arazoe, T.; Miyoshi, K.; Yamato, T.; Ogawa, T.; Ohsato, S.; Arie, T.; Kuwata, S. Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol. Bioeng. 2015, 112, 2543–2549. [Google Scholar] [CrossRef]
- Ford, K.L.; Baumgartner, K.; Henricot, B.; Bailey, A.M.; Foster, G.D. A native promoter and inclusion of an intron is necessary for efficient expression of GFP or mRFP in Armillaria mellea. Sci. Rep. 2016, 6, 29226. [Google Scholar] [CrossRef] [Green Version]
- Connolly, L.R.; Smith, K.M.; Freitag, M. The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet. 2013, 9, e1003916. [Google Scholar] [CrossRef] [Green Version]
- Lenarcic, T.; Pirc, K.; Hodnik, V.; Albert, I.; Borisek, J.; Magistrato, A.; Nurnberger, T.; Podobnik, M.; Anderluh, G. Molecular basis for functional diversity among microbial Nep1-like proteins. PLoS Pathog. 2019, 15, e1007951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Han, H.; Zhao, J.; Li, C. In-vitro and in-planta Botrytis cinerea inoculation assays for tomato. Bio-Protocol 2018, 8, e2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, M.; Fleetwood, D.J.; Johnson, R.D. Histological methods to detect early-stage plant defense responses during artificial inoculation of lolium perenne with Epichloë festucae. Bio-Protocol 2021, 11, e4013. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Cao, S.-N.; Yuan, Y.; Qin, Y.; Zhang, M.-Z.; de Figueiredo, P.; Li, G.-H.; Qin, Q.M. The pre-rRNA processing factor Nop53 regulates fungal development and pathogenesis via mediating production of reactive oxygen species. Environ. Microbiol. 2018, 20, 1531–1549. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.K.; Li, G.H.; Zhang, M.Z.; Zhang, Y.Y.; Wang, Y.Y.; Hou, J.; Yang, S.; Sun, J.; Qin, Q.M. A novel Botrytis cinerea-specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development. Mol. Plant Pathol. 2019, 20, 731–747. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Waszczak, C.; Hunter, K.; Wrzaczek, M. Bound by Fate: The Role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 2017, 29, 638–654. [Google Scholar] [CrossRef] [Green Version]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY Transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef] [Green Version]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.W.; Wu, J.; Cheng, Y.C.; Shi, J.J.; Zhang, X.J.; Shi, Y.X.; Chai, A.; Li, B.J. First report of Stemphylium lycopersici causing leaf spot on hot pepper in China. Can. J. Plant Pathol. 2019, 41, 124–128. [Google Scholar] [CrossRef]
- Yang, H.H.; Xu, X.Y.; Zhao, T.T.; Jiang, J.B.; Liu, G.; Li, J.F. First report of Stemphylium lycopersici causing gray leaf spot on eggplant in China. Plant Dis. 2017, 101, 834–835. [Google Scholar] [CrossRef]
- Kurose, D.; Misawa, T.; Suzui, T.; Ichikawa, K.; Kisaki, G.; Hoang, L.H.; Furuya, N.; Tsuchiya, K.; Tsushima, S.; Sato, T. Taxonomic re-examination of several Japanese Stemphylium strains based on morphological and molecular phylogenetic analyses. J. Gen. Plant Pathol. 2015, 81, 358–367. [Google Scholar] [CrossRef]
- Mehta, A.; Mehta, Y.R.; Rosato, Y.B. ERIC- and REP-PCR amplify non-repetitive fragments from the genome of Drechslera avenae and Stemphylium solani. FEMS Microbiol. Lett. 2002, 211, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Bailey, B.A. Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 1995, 85, 1250–1255. [Google Scholar] [CrossRef]
- Nelson, A.J. Sequence announcements. Plant Mol. Biol. 1998, 38, 911–912. [Google Scholar] [CrossRef]
- Feng, B.Z.; Li, P.Q. Molecular characterization and functional analysis of the Nep1-like protein-encoding gene from Phytophthora capsici. Genet. Mol. Res. 2013, 12, 1468–1478. [Google Scholar] [CrossRef]
- Oome, S.; Van Den Ackerveken, G. Comparative and functional analysis of the widely occurring family of Nep1-like proteins. Mol. Plant-Microbe Interact. 2014, 27, 1081–1094. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.B.; Bao, S.W.; Fang, Y.L.; Wei, L.Y.; Zhu, W.S.; Peng, Y.L.; Fan, J. An LRR-only protein promotes NLP-triggered cell death and disease susceptibility by facilitating oligomerization of NLP in Arabidopsis. New Phytol. 2021, 232, 1808–1822. [Google Scholar] [CrossRef]
- Staats, M.; VAN Baarlen, P.; Schouten, A.; VAN Kan, J.A.L. Functional analysis of NLP genes from botrytis elliptica. Mol. Plant Pathol. 2007, 8, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cobos, R.; Calvo-Peña, C.; Álvarez-Pérez, J.M.; Ibáñez, A.; Diez-Galán, A.; González-García, S.; García-Angulo, P.; Acebes, J.L.; Coque, J.J.R. Necrotic and cytolytic activity on grapevine leaves produced by Nep1-Like Proteins of Diplodia seriata. Front. Plant Sci. 2019, 10, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, S.; Grosser, K.; Voegele, R.T.; Kassemeyer, H.-H.; Fuchs, R. Identification and characterization of Nep1-Like proteins from the grapevine downy mildew pathogen Plasmopara viticola. Front. Plant Sci. 2020, 11, 65. [Google Scholar] [CrossRef]
- Jennings, J.C.; Apel-Birkhold, P.C.; Bailey, B.A.; Anderson, J.D. Induction of ethylene biosynthesis and necrosis in weed leaves by a Fusarium oxysporum protein. Weed Sci. 2000, 48, 7–14. [Google Scholar] [CrossRef]
- Santhanam, P.; van Esse, P.; Albert, I.; Faino, L.; Nürnberger, T.; Thomma, B.P.H.J. Evidence for Functional Diversification Within a Fungal NEP1-Like Protein Family. Mol. Plant-Microbe Interact. 2013, 26, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenarčič, T.; Albert, I.; Böhm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Žagar, E.; Pruitt, R. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.F.; Dias, C.V.; Cidade, L.C.; Mendes, J.S.; Pirovani, C.P.; Alvim, F.C.; Pereira, G.A.G.; Aragão, F.J.L.; Cascardo, J.C.M.; Costa, M.G.C. Expression of an oxalate decarboxylase impairs the necrotic effect induced by Nep1-like Protein (NLP) of Moniliophthora perniciosa in transgenic tobacco. Mol. Plant-Microbe Interact. 2011, 24, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Bolton, M.D. Primary Metabolism and Plant Defense—Fuel for the Fire. Mol. Plant-Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.; Oome, S.; Sander, N.; Küfner, I.; Nürnberger, T.; Van Den Ackerveken, G. Nontoxic Nep1-like proteins of the downy mildew pathogen Hyaloperonospora arabidopsidis: Repression of necrosis-inducing activity by a surface-exposed region. Mol. Plant-Microbe Interact. 2012, 25, 697–708. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, J.; Han, H.; Chen, X.; Chen, Q.; Zhao, J.; Li, C. Stemphylium lycopersici Nep1-like Protein (NLP) Is a Key Virulence Factor in Tomato Gray Leaf Spot Disease. J. Fungi 2022, 8, 518. https://doi.org/10.3390/jof8050518
Lian J, Han H, Chen X, Chen Q, Zhao J, Li C. Stemphylium lycopersici Nep1-like Protein (NLP) Is a Key Virulence Factor in Tomato Gray Leaf Spot Disease. Journal of Fungi. 2022; 8(5):518. https://doi.org/10.3390/jof8050518
Chicago/Turabian StyleLian, Jiajie, Hongyu Han, Xizhan Chen, Qian Chen, Jiuhai Zhao, and Chuanyou Li. 2022. "Stemphylium lycopersici Nep1-like Protein (NLP) Is a Key Virulence Factor in Tomato Gray Leaf Spot Disease" Journal of Fungi 8, no. 5: 518. https://doi.org/10.3390/jof8050518





