Selection of Polymorphic Patterns Obtained by RAPD-PCR through Qualitative and Quantitative Analyses to Differentiate Aspergillus fumigatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Monosporic Cultures
2.3. DNA Extraction
2.4. Amplification of the Partial Sequence of the BenA Gene
2.5. Sequence Analysis
2.6. Phylogenetic Analysis
2.7. RAPD-PCR
2.8. Qualitative and Quantitative Analyses of Polymorphic Patterns Generated by RAPD-PCR
2.8.1. Qualitative Selection
2.8.2. Quantitative Selection
2.9. Application of Mathematical Models to Identify Aspergillus spp.
3. Results
3.1. Macro- and Micromorphology
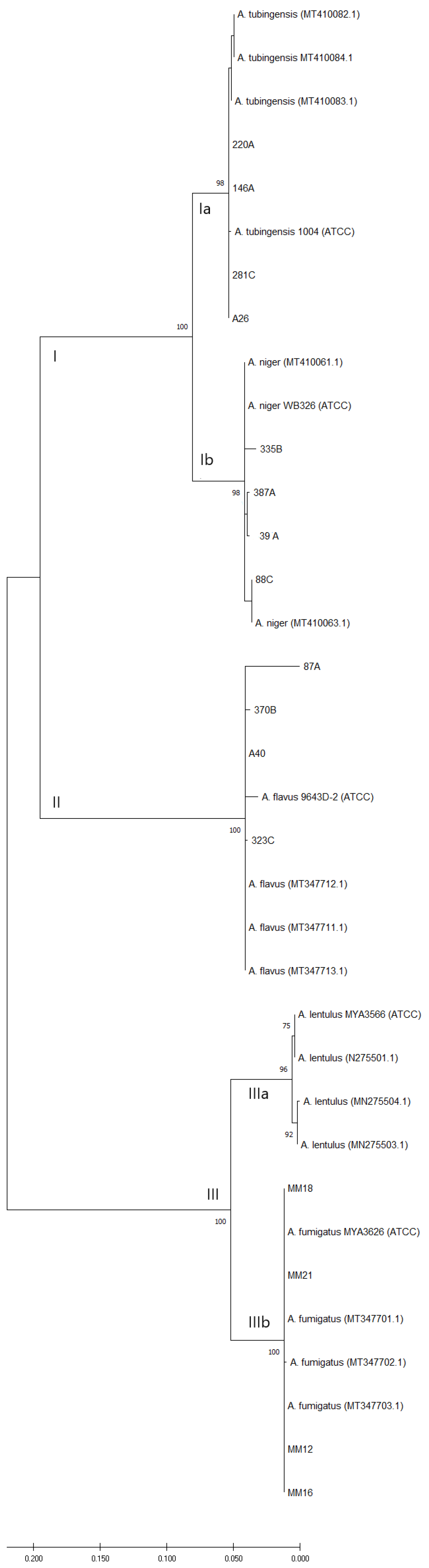
3.2. Phylogenetic Analysis
3.3. RAPD-PCR
3.4. Qualitative Selection
3.5. Quantitative Selection
3.6. Qualitative and Quantitative Comparison of the Selected Primers
3.7. Application of Mathematical Models to Identify Aspergillus spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sklenár, F.; Jurievic, Z.; Peterson, S.W.; Kolarík, M.; Novakova, A.; Flieger, M.; Stodulkova, E.; Kubátová, A.; Hubka, V. Increasing the species diversity in the Aspergillus section Nidulantes: Six novel species mainly from the indoor environment. Mycologia 2020, 112, 342–370. [Google Scholar] [CrossRef] [PubMed]
- Schabereiter-Gurtner, C.; Selitsch, B.; Rotter, M.L.; Hirschl, A.M.; Willinger, B. Development of novel real-time PCR assays for detection and differentiation of eleven medically important Aspergillus and Candida species in clinical specimens. J. Clin. Microbiol. 2007, 45, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, R.A.; Hong, S.B.; Frisvad, J.C. Old and new concepts of species differentiation in Aspergillus. Med. Mycol. 2006, 44, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinel-Ingroff, A. Clinical relevance of fungal susceptibility testing and antifungal resistance. Clin. Microbiol. Newsl. 2000, 22, 137–140. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Dilger, A.; Alcazar-Fuoli, L.; Park, S.; Mellado, E.; Perlin, D.S. Rapid detection of triazole 132 N.M. Ashtiani; et al. antifungal resistance in Aspergillus fumigatus. J. Clin. Microbiol. 2008, 46, 1200–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelenz, S.; Barnes, R.A.; Barton, R.C.; Cleverley, J.R.; Lucas, S.B.; Kibbler, C.C.; Denning, D.W.; British Society for Medical Mycology. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015, 15, 461–474. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Molina, J.V.; Abad-Díaz-de-Cerio, A.; Sueiro-Olivares, M.; Pellon, A.; Ramirez-Garcia, A.; Garaizar, J.; Pemán, J.; Hernando, F.L.; Rementeria, A. Rapid and specific detection of section Fumigati and Aspergillus fumigatus in human samples using a new multiplex real-time PCR. Diagn. Microbiol. Infect. Dis. 2014, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jewtuchowicz, V.M.; Mujica, M.T.; Malzone, M.C.; Cuesta, A.; Nastri, M.L.; Iovannitti, C.A.; Rosa, A.C. Genetic relatedness of subgingival and buccal Candida dubliniensis isolates in immunocompetent subjects assessed by RAPD-PCR. J. Oral Microbiol. 2009, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.B.; Kim, D.H.; Park, I.C.; Choi, Y.J.; Shin, H.D.; Samson, R.A. Re-identification of Aspergillus fumigatus sensu lato based on a new concept of species delimitation. J. Microbiol. 2010, 48, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Bertone, A.M.; Rosa, A.C.; Nastri, N.; Santillán, H.D.; Ariza, Y.; Iovannitti, C.A.; Jewtuchowicz, V.M. Genetic-relatedness of peri-implants and buccal Candida albicans isolates determined by RAPD-PCR. Acta Odontol. Latinoam. 2016, 29, 197–205. [Google Scholar] [PubMed]
- Kermani, F.; Shams-Ghahfarokhi, M.; Gholami-Shabani, M.; Razzaghi-Abyaneh, M. Diversity, molecular phylogeny and fingerprint profiles of airborne Aspergillus species using random amplified polymorphic DNA. World J. Microbiol. Biotechnol. 2016, 32, 96. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.A.; Alonso, V.; Manini, M.V.; Pellegrino, M.; Cavaglieri, L.R. Molecular characterization of Aspergillus fumigatus isolated from raw cow milk in Argentina: Molecular typing of A. fumigatus from raw cow milk. Int J. Food Microbiol. 2018, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thanos, M.; Schonian, G.; Meyer, W.; Schweynoch, C.; Graser, Y.; Mitchell, T.G.; Presber, W.; Tietz, H.J. Rapid identification of Candida species by DNA fingerprinting with PCR. J. Clin. Microbiol. 1996, 34, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.M.E.; Almeida-Paes, R.; Muniz, M.M.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 2011, 172, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 2nd ed.; Springer: New York, NY, USA, 1997; pp. 377–385. [Google Scholar]
- Riddell, R.W. Permanent stained mycological preparations obtained by slide culture. Mycologia 1950, 42, 265–270. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersulyte, D.; Woods, J.P.; Keath, E.J.; Goldman, W.E.; Berg, D.E. Diversity among clinical isolates of Histoplama capsulatum detected by polymerase chain reaction with arbitrary primers. J. Bacteriol. Res. 1992, 174, 7075–7079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, J.P.; Kersulyte, D.; Goldman, W.E.; Berg, D.E. Fast DNA isolation from Histoplasma capsulatum: Methodology for arbitrary primer polymerase chain reaction-based epidemiological and clinical studies. J. Clin. Microbiol. 1993, 31, 463–464. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; van Eysden, J.; Quiros, C.F. Generation of DNA-based markers in specific genome regions by two-primer RAPD reactions. PCR Methods Appl. 1995, 4, 346–351. [Google Scholar] [CrossRef]
- Fiuza, P.M.; Rodríguez, P.J.C. La regresión logística: Una herramienta versátil. Nefrología 2000, 20, 495–500. [Google Scholar]
- Fawcett, T. ROC graphs: Notes and practical considerations for researchers. Mach. Learn. 2004, 31, 1–38. [Google Scholar]
- Geiser, D.M.; Frisvad, J.C.; Taylor, J.W. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia 1998, 90, 831–845. [Google Scholar] [CrossRef]
- Duarte-Escalante, E.; Frías-De-León, M.G.; Martínez-Herrera, E.; Acosta-Altamirano, G.; Rosas de Paz, E.; Reséndiz-Sánchez, J.; Refojo, N.; Reyes-Montes, M.R. Identification of CSP types and genotypic variability of clinical and environmental isolates of Aspergillus fumigatus from different geographic origins. Microorganisms 2020, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Lasker, B.A. Evaluation of Performance of Four Genotypic Methods for Studying the Genetic Epidemiology of Aspergillus fumigatus Isolates. J. Clin. Microbiol. 2002, 40, 2886–2892. [Google Scholar] [CrossRef] [Green Version]
- Mondon, P.; Thélu, J.; Lebeau, B.; Ambroise-Thomas, P.; Grillot, R. Virulence of Aspergillus fumigatus strains investigated by random amplified polymorphic DNA analysis. J. Med. Microbiol. 1995, 42, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Lehmann, P.F.; Hamory, B.H.; Padhye, A.A.; Durry, E.; Pinner, R.W.; Lasker, B.A. Comparison of three typing methods for clinical and environmental isolates of Aspergillus fumigatus. J. Clin. Microbiol. 1995, 33, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril, C.; Acevedo, H.; Llorente, M.T.; Castaño, E. Detección in vivo mediante RAPD de alteraciones en el ADN producidas por benzo (a) pireno. Rev. Toxicol. 2004, 21, 16–22. [Google Scholar]
- Munive, M.G.R.; González, G.A.; Aguirre, D.X. ADN polimórfico amplificado al azar (RAPD) y regiones intermedias entre secuencias simples repetidas. In Herramientas Moleculares Aplicadas en Ecología: Aspectos Teóricos y Prácticos, 1st ed.; Cornejo, R.A., Serrato, D.A., Rendón, A.B., Rocha, M.G., Eds.; Instituto Nacional de Ecología y Cambio Climático: Mexico City, Mexico, 2014; pp. 117–135. [Google Scholar]
- Pavel, A.B.; Vasile, C.I. PyElph-a software tool for gel images analysis and phylogenetics. BMC Bioinform. 2012, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treviño-Castellano, M.; Rodríguez-Nóvoa, S.; Llovo-Taboada, J.; García-Zabarte, Á.; García-Riestra, C.; Regueiro-García, B.J. Uso combinado de amplificación aleatoria de polimorfismo del ADN (RAPD) y reacción en cadena de la polimerasa (touchdown PCR) en el estudio epidemiológico de Aspergillus fumigatus. Enferm. Infecc. Microbiol. Clin. 2003, 21, 472–476. [Google Scholar] [CrossRef]
- Granados, Z.S.J. Frecuencia de Neospora caninum en Bovinos Lecheros de 4 Distritos del Valle del Mantaro (Junín). Bachelor’s Thesis, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2012. [Google Scholar]
- Ausina, V.; Catalán, V.; Cercenado, E.; Pelaz, A.C. Recomendaciones de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. In Procedimientos en Microbiología Clínica; Cercenado, E., Cantón, R., Eds.; McGraw-Hill Interamericana de España: Madrid, Spain, 2005; p. 20. [Google Scholar]
- Palumbo, J.D.; O’Keeffe, T.L. Detection and discrimination of four Aspergillus section Nigri species by PCR. Lett Appl. Microbiol. 2015, 60, 188–195. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Borba, C.; Correia, J.; Vinhas, E.; Martins, A.; Costa Aguiar Alves, B.; Unkles, S.; Robertson Kinghorn, J.; Lucena-Silva, N. Genetic characterization of morphologically variant strains of Paracoccidioides brasiliensis. Memórias Inst. Oswaldo Cruz 2008, 103, 306–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Species | Isolate/Access Number | Source | Geographical Origin |
|---|---|---|---|
| A. flavus | 9343D-2 | ATCC | ND |
| 323C/OM89286 | Environmental | MX | |
| 87A/OM892858 | Environmental | MX | |
| A40/OM892868 | Environmental | MX | |
| 370B/OM892869 | Environmental | MX | |
| MM-234/MT347712.1 | Environmental | CU | |
| MM-224/MT347711.1 | Environmental | CU | |
| MM-243/MT347713.1 | Environmental | CU | |
| A. fumigatus | MYA3626 | ATCC | ND |
| MM12/OM892862 | Clinical | AR | |
| MM16/OM89286 | Clinical | AR | |
| MM18/OM892864 | Clinical | AR | |
| MM21/OM892865 | Clinical | AR | |
| MM-263/MT347701.1 | Environmental | CU | |
| MM-265/MT347702.1 | Environmental | CU | |
| MM-308/MT347703.1 | Environmental | CU | |
| A. lentulus | MYA3566 | ATCC | ND |
| A. niger | WB326 | ATCC | ND |
| 387A/OM892858 | Environmental | MX | |
| 39A/OM892859 | Environmental | MX | |
| 335B/OM892860 | Environmental | MX | |
| 88C/OM892861 | Environmental | MX | |
| MM-274/MT410062.1 | Environmental | CU | |
| MM-291/MT410063.1 | Environmental | CU | |
| A. tubingensis | 1004 | Reference strain | MX |
| 281C/OM892871 | Environmental | MX | |
| 146A/OM892872 | Environmental | MX | |
| A26/OM892870 | Environmental | MX | |
| 220A/OM892873 | Environmental | MX | |
| MM-251/MT410082 | Environmental | CU | |
| MM-267/MT410083.1 | Environmental | CU | |
| MM-342/MT410084.1 | Environmental | CU |
| Species | Access Number |
|---|---|
| A. niger | MT410061.1 MT410062.1 MT410063.1 |
| A. tubingensis | MT410082.1 MT410083.1 MT410083.1 |
| A. flavus | MT347712.1 MT347711.1 MT347711.1 |
| A. fumigatus | MT347701.1 MT347702.1 MT347703.1 |
| A. lentulus | MN275504.1 MN275503.1 N275501.1 |
| Primer | Sequence 5′-3′ |
|---|---|
| 1281 | AACGCGCAAC |
| 1283 | CGGATCCCCA |
| R108 | GTATTGCCCT |
| 1253 | GTTTCCGCCC |
| OPA-19 | CAAACGTCGG |
| OPA-16 | AGCCAGCGAA |
| OPB-12 | CCTTGACGCA |
| OPA-17 | GACCGCTTGT |
| OPA-15 | TTCCGAACCC |
| OPF-05 | CCGAATTCCC |
| OPA-08 | GTGACGTAGG |
| OPC-07 | GTCCCGATGA |
| OPG-01 | CTACGGAGGA |
| OPG-03 | GAGCCCTCCA |
| OPG-13 | CTCTCCGCCA |
| OPG-15 | ACTGGGACTC |
| OPC-06 | GAACGGACTC |
| OPG-05 | CTGAGACGGA |
| OPM-12 | GGGACGTTGG |
| OPH-03 | AGACGTCCAC |
| OPG-07 | GAACCTGCGG |
| OPH-18 | GAATCGGCCA |
| OPF-01 | ACGGATTCTG |
| OPF-07 | CCGATATCCC |
| OPF-09 | CCAAGCTTCC |
| OPE-02 | GGTGCGGGAA |
| P54 | GGCGATTTTTGCCG |
| P160 | CATGGCCACC |
| PELF | ATATCATCGAAGCCGC |
| B-04 | TGCCATCAGT |
| OPH-17 | CACTCTCCTC |
| T3B | AGGTCGCGGGTTCGAATCC |
| OPA-02 | TGCCGAGCTG |
| OPA-03 | AGTCAGCCAC |
| Species-Specific Primers | Identified Species | Number of Bands Generated | Molecular Size (pb) |
|---|---|---|---|
| OPA-19 | A. fumigatus | 3–5 | 200–2400 |
| 1253 | A. niger | 13 | 190–16,002 |
| OPA-02 | A. tubingensis | 2 | 500–1600 |
| P54 | A. flavus | 13 | 190–2300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Ledezma, O.E.; Castro-Fuentes, C.A.; Duarte-Escalante, E.; Frías-De-León, M.G.; Reyes-Montes, M.d.R. Selection of Polymorphic Patterns Obtained by RAPD-PCR through Qualitative and Quantitative Analyses to Differentiate Aspergillus fumigatus. J. Fungi 2022, 8, 296. https://doi.org/10.3390/jof8030296
Valencia-Ledezma OE, Castro-Fuentes CA, Duarte-Escalante E, Frías-De-León MG, Reyes-Montes MdR. Selection of Polymorphic Patterns Obtained by RAPD-PCR through Qualitative and Quantitative Analyses to Differentiate Aspergillus fumigatus. Journal of Fungi. 2022; 8(3):296. https://doi.org/10.3390/jof8030296
Chicago/Turabian StyleValencia-Ledezma, Omar E., Carlos A. Castro-Fuentes, Esperanza Duarte-Escalante, María Guadalupe Frías-De-León, and María del Rocío Reyes-Montes. 2022. "Selection of Polymorphic Patterns Obtained by RAPD-PCR through Qualitative and Quantitative Analyses to Differentiate Aspergillus fumigatus" Journal of Fungi 8, no. 3: 296. https://doi.org/10.3390/jof8030296






