Abscisic Acid May Play a Critical Role in the Moderating Effect of Epichloë Endophyte on Achnatherum inebrians under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Measurement Protocols
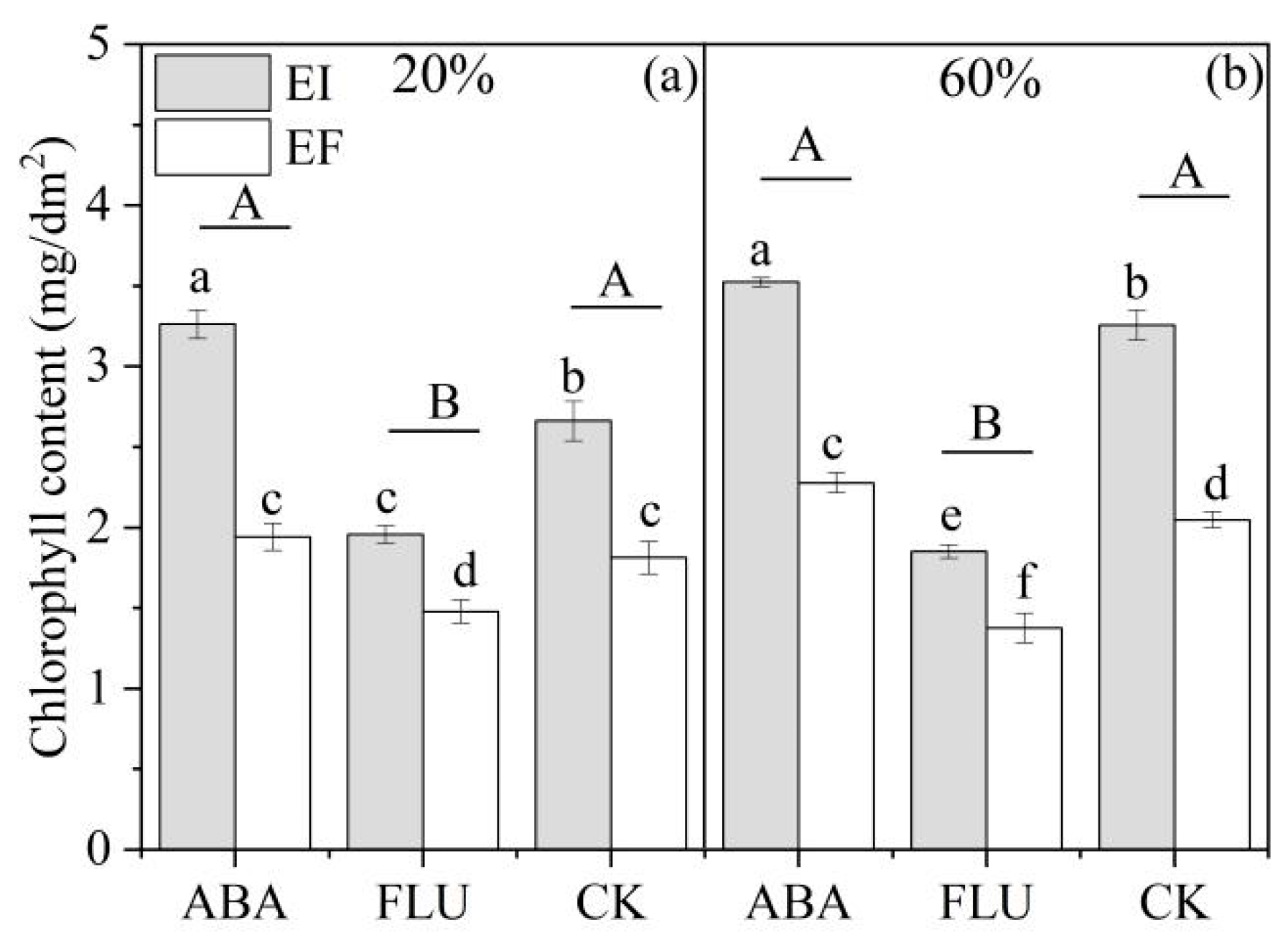
2.3.1. Chlorophyll Content
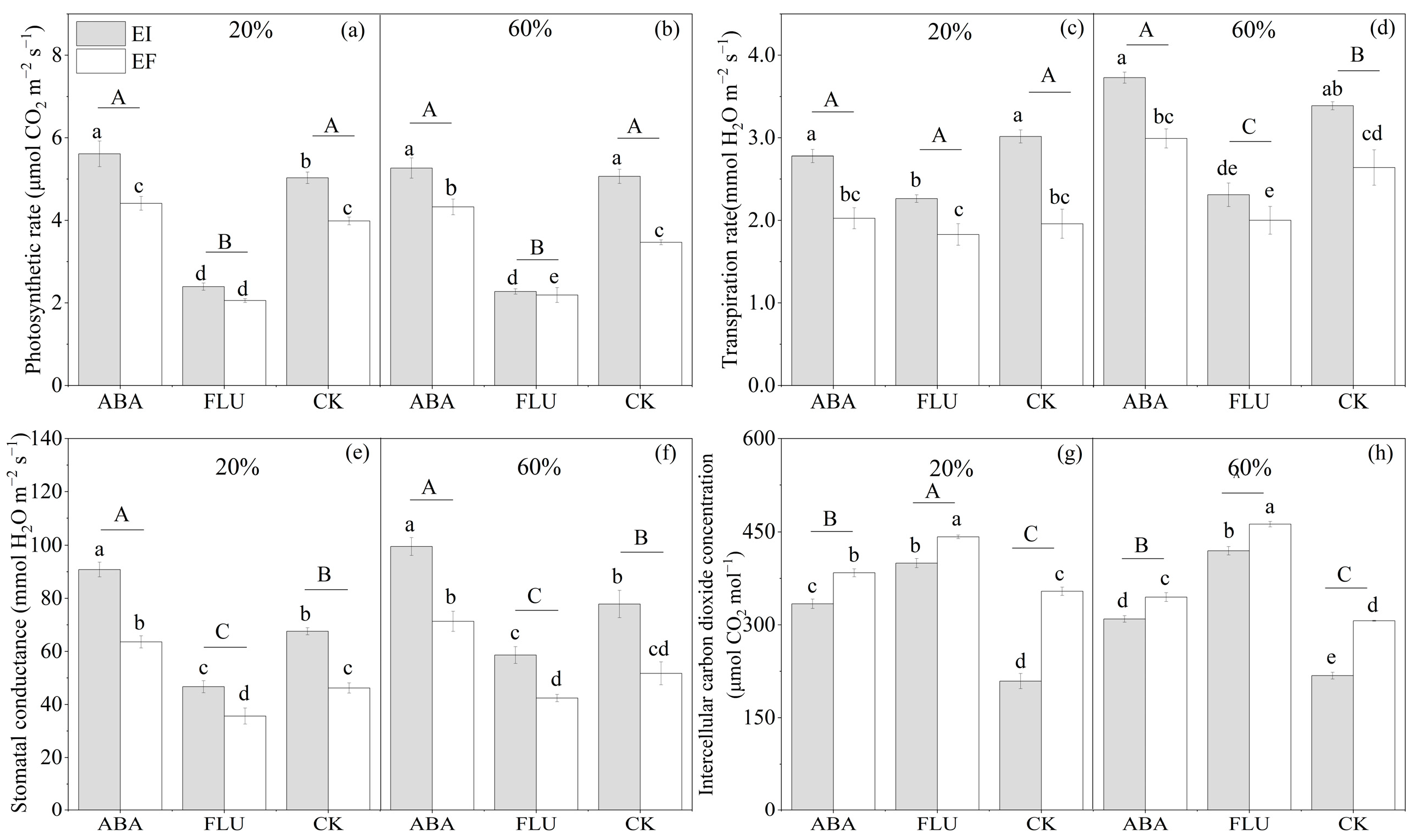
2.3.2. Photosynthetic Indexes
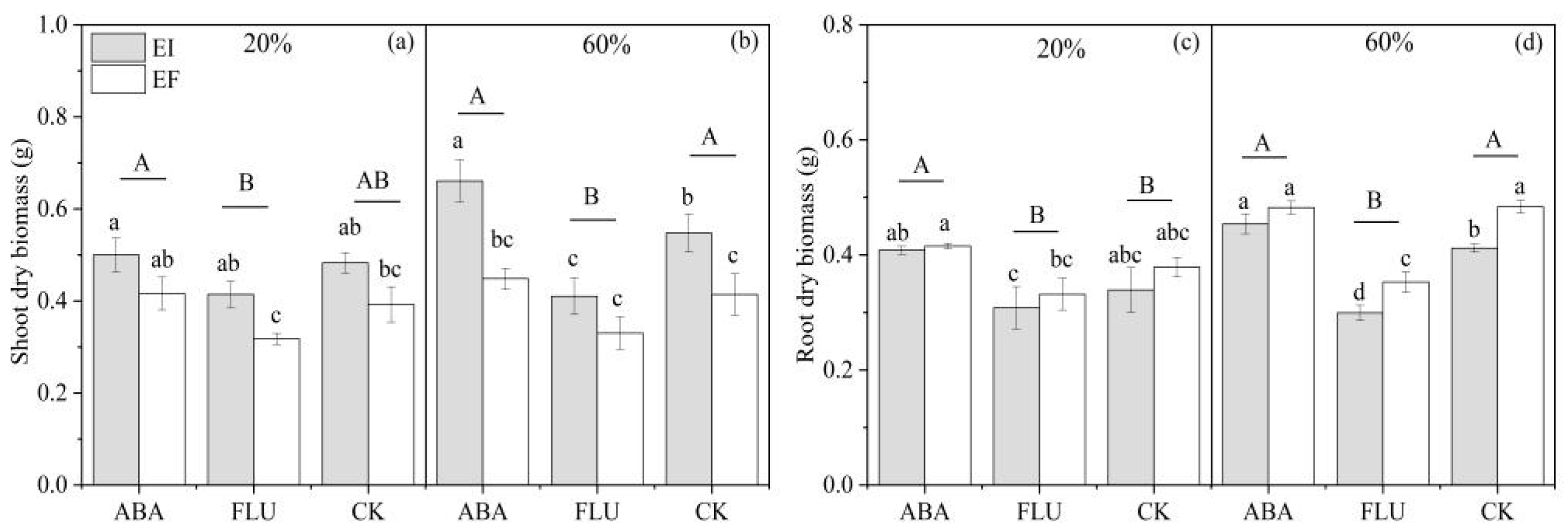
2.3.3. Biomass Production and Growth
2.3.4. Statistical Analysis
3. Results
3.1. Plant Growth Parameters of EI and EF Plants
3.1.1. Plant Height and Tiller Number
3.1.2. Biomass
3.2. Photosynthetic Indexes of EI and EF Plants
3.2.1. Chlorophyll Content
3.2.2. Photosynthetic Rate
3.2.3. Transpiration Rate
3.2.4. Stomatal Conductance
3.2.5. Intercellular Carbon Dioxide Concentration
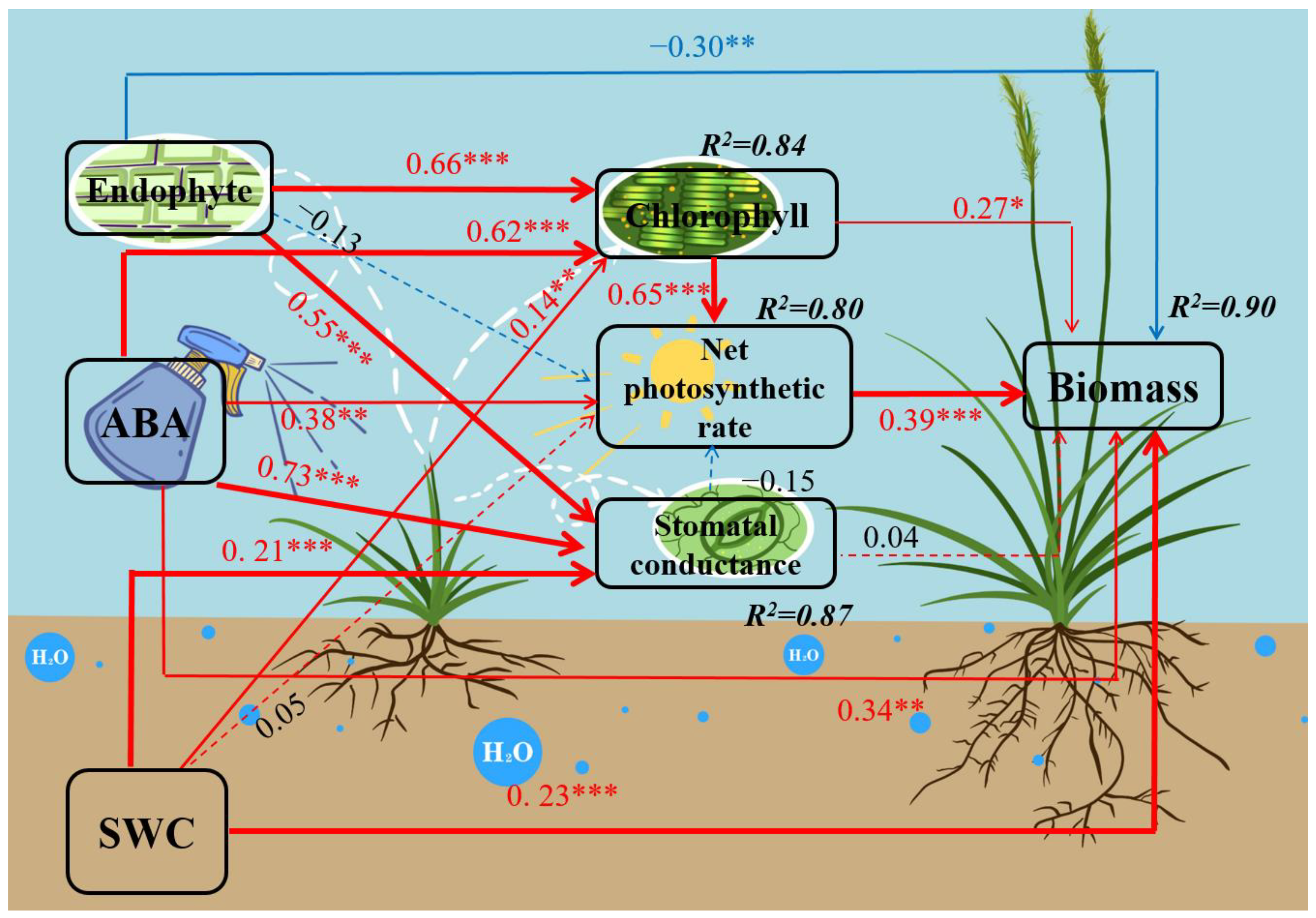
3.3. SEM for the Interactive Effects of Endophyte, Water and ABA on DHG Biomass
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop. Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New. Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Lowman, S.; Kim-Dura, S.; Mei, C.; Nowak, J. Strategies for enhancement of switchgrass (Panicum virgatum L.) performance under limited nitrogen supply based on utilization of N-fixing bacterial endophytes. Plant Soil 2016, 405, 47–63. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal. Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Johnson, L.J.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; Tapper, B.A. The exploitation of epichloae endophytes for agricultural benefit. Fungal. Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Li, X.Z.; Nan, Z.B. Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Molina-Montenegro, M.A.; Ramos, P.; Ghersa, C.M. Getting ready for the ozone battle: Vertically transmitted fungal endophytes have transgenerational positive effects in plants. Plant Cell. Environ. 2021, 44, 2716–2728. [Google Scholar] [CrossRef]
- Saedi, T.; Mosaddeghi, M.R.; Sabzalian, M.R.; Zarebanadkouki, M. Effect of Epichloë fungal endophyte symbiosis on tall fescue to cope with flooding-derived oxygen-limited conditions depends on the host genotype. Plant Soil 2021, 468, 353–373. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, L.M.; Popay, A.J.; Glare, T.R.; Finch, S.C.; Cave, V.M.; Rostás, M. Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass. J. Pest Sci. 2022, 95, 263–277. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Paul, V.H.; Dapprich, P.A. new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon. 2004, 90, 141–147. [Google Scholar]
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000. [Google Scholar]
- Zhang, X.X.; Nan, Z.B.; Li, C.J.; Gao, K. Cytotoxic Effect of ergot alkaloids in Achnatherum inebrians infected by the Neotyphodium gansuense endophyte. J. Agric. Food Chem. 2014, 62, 7419–7422. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.C.; Li, C.J.; Nan, Z.B.; Li, F.D. Effects of feeding drunken horse grass infected with Epichloë gansuensis endophyte on animal performance, clinical symptoms and physiological parameters in sheep. BMC Vet. Res. 2017, 13, 223. [Google Scholar] [CrossRef] [Green Version]
- Li, C.J.; Nan, Z.B.; Zhang, C.; Zhang, C.; Zhang, Y. Effects of drunken horse grass infected with endophyte on Chinese rabbit. J. Agric. Sci. Technol. 2009, 11, 90–96. [Google Scholar]
- Zhang, X.X.; Li, C.J.; Nan, Z.B.; Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed. Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- He, Y.L.; Chen, T.X.; Zhang, H.J.; White, J.F.; Li, C.J. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2021, 41, 2122–2137. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal. Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.B. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.Z.; White, J.F.; Wei, X.K.; Li, C.J. Epichloë endophyte improves ergot disease resistance of host (Achnatherum inebrians) by regulating leaf senescence and photosynthetic capacity. J. Plant Growth Regul. 2021, 41, 808–817. [Google Scholar] [CrossRef]
- Chen, N.; He, R.L.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Zhang, X.X. Research progress of improved resistance of the grass to the heave mental stress by endophyte. Pratacultural. Sci. 2014, 31, 1466–1474. [Google Scholar]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, X.X.; Nan, Z.B. The fungal endophyte Epichloë gansuensis increases NaCl-tolerance in Achnatherum inebrians through enhancing the activity of plasma membrane H+-ATPase and glucose-6-phosphate dehydrogenase. Sci. China Life Sci. 2021, 64, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, J.F.; Hou, W.P.; Malik, K.; Zhao, C.Z.; Niu, X.L.; Liu, Y.L.; Huang, R.; Li, C.J.; Nan, Z.B. Elucidating the Molecular Mechanisms by which Seed-Borne Endophytic Fungi, Epichloë gansuensis, Increases the Tolerance of Achnatherum inebrians to NaCl Stress. Int. J. Mol. Sci. 2021, 22, 13191. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.; Ávila, R.T.; Morais, L.E.; Martins, S.C.; Nobres, P.; Patreze, C.M.; Ferreira, F.M.; Araújo, W.L.; Fernie, E.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Sahay, S.; Torres, E.D.; Arratia, L.R.; Gupta, M. Photosynthetic activity and RAPD profile of polyethylene glycol treated B. juncea L. under nitric oxide and abscisic acid application. J. Biotechnol. 2020, 313, 29–38. [Google Scholar] [CrossRef]
- Hasanagic, D.; Koleska, I.; Kojic, D.; Vlaisavljevic, S.; Janjic, N.; Kukavica, B. Long term drought effects on tomato leaves: Anatomical, gas exchange and antioxidant modifications. Acta Physiol. Plant 2020, 42, 121. [Google Scholar]
- Wilkinson, S.; Davies, W.J. Aba-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell. Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, C.; Reinoso, H.; Cohen, A.; Luna, C.; Tommasino, E.; Castillo, C.; Bottini, R. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul. 2010, 29, 366–374. [Google Scholar] [CrossRef]
- Xu, W.B.; Li, M.M.; Lin, W.H.; Nan, Z.B.; Tian, P. Effects of Epichloë sinensis endophyte and host ecotype on physiology of festuca sinensis under different soil moisture conditions. Plants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar]
- Leung, J.; Giraudat, J. Abscisic acid signal transduction. Annu. Rev. Plant Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Bauerle, W.L.; Inman, W.W.; Dudley, J.B. Leaf abscisic acid accumulation in response to substrate water content: Linking leaf gas exchange regulation with leaf abscisic acid concentration. J. Am. Soc. Hortic. Sci. 2006, 131, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Sawada, Y.; Takahashi, H.; Okamoto, M.; Ikegami, K.; Koiwai, H.; Nambara, E. Drought induction of arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Verslues, P.E.; Zhu, J.K. Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc. Trans. 2005, 33, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell. Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.Y.; Guo, Z.F.; Lin, L. Effects of abscisic acid application on photosynthesis and photochemistry of Stylosanthes guianensis under chilling stress. Plant Growth Regul. 2006, 48, 195–199. [Google Scholar]
- Fotovat, R.; Valizadeh, M.; Toorehi, M. Association between water-use efficiency components and total chlorophyll content (SPAD) in wheat (Triticum aestivum L.) under well-watered and drought stress conditions. J. Food Agric. Environ. 2007, 5, 225–227. [Google Scholar]
- De Battista, J.P.; Bouton, J.H.; Bacon, C.W.; Siegel, M.R. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agron. J. 1990, 82, 651–654. [Google Scholar] [CrossRef]
- Dupont, P.Y.; Eaton, C.J.; Wargent, J.J.; Fechtner, S.; Solomon, P.; Schmid, J.; Cox, M.P. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 2015, 208, 1227–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, B.; Green, K.; Berry, D. The fine balance between mutualism and antagonism in the Epichloë festucae–grass symbiotic interaction. Curr. Opin. Plant Biol. 2018, 44, 32–38. [Google Scholar] [CrossRef]
- Li, N.N.; Zhao, Y.F.; Xia, C.; Zhong, R.; Zhang, X.X. Effects of thiophanate methyl on seed borne Epichloë fungal endophyte of Achnatherum inebrians. Pratacultural. Sci. 2016, 33, 1306–1314. [Google Scholar]
- Li, C.J.; Nan, Z.B.; Liu, Y. Methodology of endophyte detection of drunken horse grass. Edible Fungi China 2008, 27, 16–19. [Google Scholar]
- Sansberro, P.A.; Mroginski, L.A.; Bottini, R. Foliar sprays with ABA promote growth of Ilex paraguariensis by alleviating diurnal water stress. Plant Growth Regul. 2004, 42, 105–111. [Google Scholar] [CrossRef]
- Xie, J.J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, Y.B.; Jiang, D. Effect of exogenous application of abscisic acid and jasmonic acid at seedling stage on post-anthesis drought stress and physiological mechanism in Wheat. Triticeae Crops 2018, 38, 221–229. [Google Scholar]
- Ye, W.; Hu, S.; Wu, L.; Ge, C.; Cui, Y.; Chen, P.; Xu, J.; Dong, G.; Guo, L.; Qian, Q. Fine mapping a major QTL qFCC7L for chlorophyll content in rice (Oryza sativa L.) cv. PA64s. Plant Growth Regul. 2017, 81, 81–90. [Google Scholar] [CrossRef]
- Kane, K.H. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ. Exp. Bot. 2011, 71, 337–344. [Google Scholar]
- Hume, D.E.; Ryan, G.D.; Gibert, A.; Helander, M.; Sabzalian, M.R. Epichloë fungal endophytes for grassland ecosystems. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–305. [Google Scholar]
- Dastogeer, K.M.G. Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: A meta-analysis. Planta 2018, 248, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [PubMed] [Green Version]
- Tardieu, F.; Granier, C.; Muller, B. Modelling leaf expansion in a fluctuating environment: Are changes in specific leaf area a consequence of changes in expansion rate? New Phytol. 1999, 143, 33–44. [Google Scholar] [CrossRef]
- Ren, A.Z.; Gao, Y.B.; Wang, W. Photosynthetic pigments and photosynthetic products of endophyte-infection andendophyte-free Lolium perenne L. under drought stress conditions. Acta Ecologica. Sin. 2005, 25, 225–231. [Google Scholar]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar]
- Xu, Z.; Ren, J.; Tian, Y.; Mi, N. Effect of exogenous ABA photosynthetic characteristics of lolium perenne under drought stress. Acta Agrestia. Sin. 2019, 27, 1243–1249. [Google Scholar]
- Brodribb, T.J.; McAdam, S.A.M. Evolution of the stomatal regulation of plant water content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [PubMed] [Green Version]
- Malinowski, D.P.; Leuchtmann, A.; Schmidt, D.; Nösberger, J. Symbiosis with Neotyphodium uncinatum endophyte may increase the competitive ability of meadow fescue. Agron. J. 1997, 89, 833–839. [Google Scholar]
- Manzur, M.E.; Garello, F.A.; Omacini, M. Endophytic fungi and drought tolerance: Ecophysiological adjustment in shoot and root of an annual mesophytic host grass. Funct. Plant Biol. 2022, 49, 272–282. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Davitt, A.J.; Chen, C.; Rudgers, J.A. Understanding contextdependency in plant-microbe symbiosis: The influence ofabiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environ. Exp. Bot. 2011, 71, 137–145. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Jagadeesan, H.; Kumar, S.R.; Ramalingam, S. Molecular mechanisms in grass-Epichloë interactions: Towards endophyte driven farming to improve plant fitness and immunity. World J. Microb. Biot. 2020, 36, 92. [Google Scholar] [CrossRef] [PubMed]
- Latch, G.C.M.; Christensen, M.J.; Samuels, G.J. Five endophytes of Lolium and Festuca in New Zealand. Mycotaxon 1984, 20, 535–550. [Google Scholar]
- Christensen, M.J.; Voisey, C.R. The biology of the endophyte/grass partnership. NZGA Res. Pract. Ser. 2006, 13, 123–133. [Google Scholar] [CrossRef]
- Li, N.N. Effects of Interaction of Endophyte and Blumeria graminis on the Achnatherum inebrians in Different Water Conditions; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Yang, S.; Meng, L. The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrió-Seguí, À.; Romero, P.; Sanz, A.; Peñarrubia, L. Interaction between ABA signaling and copper homeostasis in Arabidopsis thaliana. Plant Cell. Physiol. 2016, 57, 1568–1582. [Google Scholar] [PubMed] [Green Version]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Spollen, W.G.; LeNoble, M.E.; Samuels, T.D.; Bernstein, N.; Sharp, R.E. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000, 122, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Black, C.R.; Taylor, I.B.; Roberts, J.A. Soil compaction. A role for ethylene in regulating leaf expansion and shoot growth in tomato? Plant Physiol. 1999, 121, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Black, C.R.; Taylor, I.B.; Roberts, J.A. Does an antagonistic relationship between ABA and ethylene mediate shoot growth when tomato (Lycopersicon esculentum Mill.) plants encounter compacted soil? Plant Cell. Environ. 2000, 23, 1217–1226. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Xu, W.F.; Jia, L.G.; Shi, W.M.; Liang, J.S.; Zhou, F.; Li, Q.F.; Zhang, J.H. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Cai, L.; Feng, Y. Effects of exogenous ABA on the physiological characteristics and chlorophyll fluorescence of Gynura cusimbua seedlings under drought stress. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 615, p. 012089. [Google Scholar]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J. Plant Res. 2009, 122, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jensen, C.R.; Shahanzari, A.; Andersen, M.N.; Jacobsen, S. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Li, X.S.; Han, L.B.; Li, D.Y.; Song, G.L. Epichloë endophyte infection improved drought and heat tolerance of tall fescue through altered antioxidant enzyme activity. Eur. J. Hortic. Sci. 2017, 82, 90–97. [Google Scholar] [CrossRef]
- Bao, G.S.; Song, M.L.; Wang, Y.Q.; Liu, J.; Wang, H.S. Interactive effects of different densities of Pedicularis kansuensis parasitism and Epichloë endophy infection on the endogenous hormone levels and alkaloid contents of Stipa purpurea. Acta Prataculturae. Sin. 2020, 29, 147–156. [Google Scholar]
- Cui, X.L.; Zhang, X.X.; Shi, L.L.; Christensen, M.J.; Nan, Z.B.; Xia, C. Effects of Epichloë endophyte and transgenerational effects on physiology of Achnatherum inebrians under drought stress. Agriculture 2022, 12, 761. [Google Scholar] [CrossRef]

| Treatments | df | Plant Height | Tiller Number | Shoot Dry Biomass | Root Dry Biomass | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| W | 1 | 0.322 | 0.573 | 20.281 | <0.000 | 5.684 | 0.021 | 17.957 | <0.000 |
| A | 2 | 114.111 | <0.000 | 76.504 | <0.000 | 16.278 | <0.000 | 33.640 | <0.000 |
| E | 1 | 185.884 | <0.000 | 16.133 | <0.000 | 33.324 | <0.000 | 9.857 | 0.003 |
| W × A | 2 | 4.709 | 0.014 | 11.437 | <0.000 | 1.774 | 0.181 | 4.063 | 0.023 |
| W × E | 1 | 1.787 | 0.188 | 3.333 | 0.074 | 1.630 | 0.208 | 1.377 | 0.246 |
| A × E | 2 | 13.561 | <0.000 | 0.533 | 0.59 | 0.742 | 0.481 | 0.852 | 0.433 |
| W × A × E | 2 | 1.302 | 0.281 | 0.533 | 0.59 | 1.093 | 0.343 | 0.021 | 0.980 |
| Treatments | df | Chlorophyll Content | Photosynthetic Rate | Transpiration Rate | Stomatal Conductance | Intercellular Dioxide Concentration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| W | 1 | 20.383 | <0.001 | 2.456 | 0.124 | 0.016 | 0.901 | 1.383 | 0.245 | 5.150 | 0.028 |
| A | 2 | 205.192 | <0.001 | 294.803 | <0.001 | 15.975 | <0.001 | 23.799 | <0.001 | 252.561 | <0.001 |
| E | 1 | 425.305 | <0.001 | 82.563 | <0.001 | 73.141 | <0.001 | 59.181 | <0.001 | 225.034 | <0.001 |
| W × A | 2 | 12.189 | <0.001 | 0.679 | 0.512 | 7.651 | <0.001 | 8.840 | <0.001 | 14.606 | <0.001 |
| W × E | 1 | 1.085 | 0.303 | 0.005 | 0.944 | 4.648 | <0.001 | 3.850 | 0.056 | 6.923 | 0.011 |
| A × E | 2 | 27.756 | <0.001 | 12.402 | <0.001 | 44.930 | <0.001 | 46.957 | <0.001 | 12.100 | <0.001 |
| W × A × E | 2 | 2.223 | 0.119 | 1.989 | 0.148 | 3.324 | 0.044 | 2.883 | 0.066 | 25.648 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; He, W.; Christensen, M.J.; Yue, J.; Zeng, F.; Zhang, X.; Nan, Z.; Xia, C. Abscisic Acid May Play a Critical Role in the Moderating Effect of Epichloë Endophyte on Achnatherum inebrians under Drought Stress. J. Fungi 2022, 8, 1140. https://doi.org/10.3390/jof8111140
Cui X, He W, Christensen MJ, Yue J, Zeng F, Zhang X, Nan Z, Xia C. Abscisic Acid May Play a Critical Role in the Moderating Effect of Epichloë Endophyte on Achnatherum inebrians under Drought Stress. Journal of Fungi. 2022; 8(11):1140. https://doi.org/10.3390/jof8111140
Chicago/Turabian StyleCui, Xuelian, Wen He, Michael John. Christensen, Jinfeng Yue, Fanbin Zeng, Xingxu Zhang, Zhibiao Nan, and Chao Xia. 2022. "Abscisic Acid May Play a Critical Role in the Moderating Effect of Epichloë Endophyte on Achnatherum inebrians under Drought Stress" Journal of Fungi 8, no. 11: 1140. https://doi.org/10.3390/jof8111140








