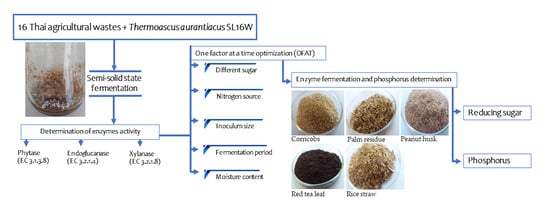
Valorization of Lignocellulosic Wastes to Produce Phytase and Cellulolytic Enzymes from a Thermophilic Fungus, Thermoascus aurantiacus SL16W, under Semi-Solid State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Inoculum Preparation
2.2. Lignocellulosic Wastes
2.3. Effect of Various Substrates on Phytase, Endoglucanase, and Xylanase Production by Semi-Solid State Fermentation (SSSF) Procedure
2.4. Extraction and Determination of Enzyme Activity
2.4.1. Enzyme Extraction
2.4.2. Phytase Assay
2.4.3. Endoglucanase Assay
2.4.4. Xylanase Assay
2.5. One Factor at a Time Optimization (OFAT) for Phytase, Endoglucanase and Xylanase Production
2.5.1. Effect of Different Sugars
2.5.2. Effect of Different Nitrogen Sources
2.5.3. Effect of Amount of Fungal Inoculum
2.5.4. Effect of Fermentation Period
2.5.5. Effect of Moisture Content
2.6. Enzyme Fermentation
2.7. Statistical Analysis
3. Results
3.1. Effect of Various Substrates on Phytase, Endoglucanase and Xylanase Production under SSSF
3.2. One Factor at a Time Optimization (OFAT) for Phytase, Endoglucanase and Xylanase Production
3.2.1. Effect of Different Sugars and Nitrogen Sources
3.2.2. Effect of Amount of Fungal Inoculum
3.2.3. Effect of Fermentation Period
3.2.4. Effect of Moisture Content
3.3. Enzyme Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briens, C.; Piskorz, J.; Berruti, F. Biomass valorization for fuel and chemicals production--a review. Int. J. Chem. React. Eng. 2008, 6, 1–49. [Google Scholar] [CrossRef]
- Mtui, G.Y. Lignocellulolytic enzymes from tropical fungi: Types, substrates and applications. Sci. Res. Essays 2012, 7, 1544–1555. [Google Scholar]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavya, V.; Padmavathi, T. Optimization of growth conditions for xylanase production by Aspergillus niger in solid state fermentation. Pol. J. Microbiol. 2009, 58, 125–130. [Google Scholar] [PubMed]
- Bujna, E.; Rezessy-Szabó, J.M.; Nguten, D.V.; Nguyen, D.Q. Production and some properties of extracellular phytase from Thermomyces lanuginosus IMI 096218 on rice flour as substrate. Mycosphere 2016, 7, 1576–1587. [Google Scholar] [CrossRef]
- Simpson, C.J.; Wise, A. Binding of zinc and calcium to inositol phosphates (phytate) in vitro. Br. J. Nutr. 1990, 64, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Konietzny, U.; Greiner, R. Phytic Acid: Nutritional Impact. In Encyclopedia of Food Science and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Elsevier: London, UK, 2003; pp. 4555–4563. [Google Scholar]
- Vohra, A.; Kaur, P.; Satyanarayana, T. Production, characteristics and applications of the cell-bound phytase of Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 51–55. [Google Scholar] [CrossRef]
- Mullaney, E.J.; Ullah, A.H.J. The term phytase comprises several different classes of enzymes. Biochem. Biophys. Res. Commun. 2003, 312, 179–184. [Google Scholar] [CrossRef]
- Gaind, S.; Singh, S. Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC6720. Int. Biodeter. Biodegr. 2015, 99, 15–22. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Greiner, R. Enzymes used in animal feed: Leading technologies and forthcoming developments. In Functional Polymers in Food Science; Cirillo, G., Spizzirri, G., Lemma, F., Eds.; Wiley: Calabria, Italy, 2014; pp. 47–73. [Google Scholar]
- Erpel, F.; Restovic, F.; Arce-Johnson, P. Development of phytase-expressing Chlamydomonas reinhardtii for monogastric animal nutrition. BMC Biotechnol. 2016, 16, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Eeckhout, W.; De Paepe, M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 1994, 47, 19–29. [Google Scholar] [CrossRef]
- Jatuwong, K.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Bioprocessing of agricultural residues as substrates and optimal conditions for phytase production of chestnut mushroom, Pholiota adiposa, in solid state fermentation. J. Fungi 2020, 6, 384. [Google Scholar] [CrossRef]
- Vats, P.; Banerjee, U.C. Studies on the production of phytase by a newly isolated strain of Aspergillus niger var teigham obtained from rotten wood logs. Process Biochem. 2002, 38, 211–217. [Google Scholar] [CrossRef]
- Brienzo, M.; Arantes, V.; Milagres, A.M. Enzymology of the thermophilic ascomycetous fungus Thermoascus aurantiacus. Fungal Biol. Rev. 2008, 22, 120–130. [Google Scholar] [CrossRef]
- McClendon, S.D.; Batth, T.; Petzold, C.J.; Adams, P.D.; Simmons, B.A.; Singer, S.W. Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnol. Biofuels. 2012, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongbuntad, W.; Saenphet, K.; Khanongnuch, C.; Lumyong, S. Determination of toxic effects of crude xylanase derived from Thermoascus aurantiacus SL16W by hematology and blood biochemistry in albino rats. Pak. J. Biol. Sci. 2006, 9, 2255–2260. [Google Scholar] [CrossRef]
- Kongbuntad, W.; Khanongnuch, C.; Lumyong, S. Efficacy of xylanase supplementation produced from Thermoascus aurantiacus SL16W in diet on Thai native chicken performance. Int. J. Poult. Sci. 2006, 5, 463–469. [Google Scholar]
- Chawachart, N.; Kasinubon, Y.; Khanongnuch, C.; Leisola, M.; Lumyong, S. Evaluation of xylanase production by a thermophillic fungus Thermoascus aurantiacus SL16W using statistic experimental designs and the arabinose inductive effect. Chiang Mai J. Sci. 2014, 41, 48–59. [Google Scholar]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; des Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Gupte, A.; Madamwar, D. Production of cellulolytic enzymes by coculturing of Aspergillus ellipticus and Aspergillus fumigatus grown on bagasse under solid state fermentation. Appl. Biochem. Biotechnol. 1997, 62, 267–274. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Nandi, B. Optimization of cellulase production by Trichoderma reesei ATCC 26921 using a simplified medium on water hyacinth biomass. J. Sci. Ind. Res. 1999, 58, 107–111. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Economou, C.N.; Makri, A.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour. Technol. 2010, 101, 1385–1388. [Google Scholar] [CrossRef]
- Gonzalez, J.C.; Medina, S.C.; Rodriguez, A.; Osma, J.F.; Alméciga-Díaz, C.J.; Sànchez, O.F. Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro-industrial wastes. PLoS ONE 2013, 8, e73721. [Google Scholar] [CrossRef]
- Gupta, A.; Jana, A.K. Effects of wheat straw solid contents in fermentation media on utilization of soluble/insoluble nutrient, fungal growth and laccase production. 3 Biotech 2018, 8, 30305998. [Google Scholar] [CrossRef]
- Phytase from Aspergillus niger Expressed in A. niger. Prepared at the 76th JECFA and Published in FAO JECFA Monographs 13. 2012. Available online: http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph13/additive-528-m13.pdf (accessed on 2 March 2020).
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1998. [Google Scholar]
- Jatuwong, K.; Suwannarach, N.; Kumla, J.; Penkhrue, W.; Kakumyan, P.; Lumyong, S. Bioprocess for production, characteristics, and biotechnological applications of fungal phytases. Front. Microbiol. 2020, 11, 188. [Google Scholar] [CrossRef]
- Barrios-González, J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Machado, I.; Teixeira, J.A.; Rodríguez-Couto, S. Semi-solid-state fermentation: A promising alternative for neomycin production by the actinomycete Streptomyces fradiae. J. Biotechnol. 2013, 165, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusharyoto, W.; Sari, M.; Ridwanuloh, A.M. Effect of culture conditions on phytase production by Aspergillus ficuum in solid-state fermentation using rice bran as substrate. Ann. Bogor. 2009, 13, 1–11. [Google Scholar]
- Kanpiengjai, A.; Unban, K.; Pratanaphon, R.; Khanongnuch, C. Optimal medium and conditions for phytase production by thermophilic bacterium, Anoxybacillus sp. MHW14. FAB J. 2013, 1, 172–189. [Google Scholar]
- Supreeth, S.; Arpitha, C.; Agarwal, A.; More, S. Isolation, optimization and production of two novel bacterial phytases from Aeromonas spp. using rice bran. BioTechnol. Indian J. 2015, 11, 55765332. [Google Scholar]
- Chadha, B.; Harmeet, G.; Mandeep, M.; Saini, H.S.; Singh, N. Phytase production by the thermophilic fungus Rhizomucor pusillus. World J. Microbiol. Biotechnol. 2004, 20, 105–109. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Tomes, G.J.; Roopesh, K.; Szakacs, G.; Nagy, V.; Soccol, C.R.; Pandey, A. Thermostable phytase production by Thermoascus aurantiacus in submerged fermentation. Appl. Biochem. Biotechnol. 2004, 118, 205–214. [Google Scholar] [CrossRef]
- Shivanna, G.B.; Venkateswaran, G. Phytase Production by Aspergillus niger CFR 335 and Aspergillus ficuum SGA 01 through submerged and solid-state fermentation. Sci. World J. 2014, 2014, 392615. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.B.E.; De Freitas, A.J.; Souza, F.F.e.; Salgado, R.L.; Guimarães, V.M.; Pereira, F.A.; Eller, M.R. Production of fungal phytases from agroindustrial byproducts for pig diets. Sci. Rep. 2019, 9, 9256. [Google Scholar] [CrossRef]
- Brijwani, K.; Oberoi, H.S.; Vadlani, P.V. Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 2010, 45, 120–128. [Google Scholar] [CrossRef]
- Coffman, A.M.; Li, Q.; Ju, L.-K. Effect of natural and pretreated soybean hulls on enzyme production by Trichoderma reesei. J. Am. Oil Chem. Soc. 2014, 91, 1331–1338. [Google Scholar] [CrossRef]
- Jain, K.K.; Dey, T.B.; Kumar, S.; Kuhad, R.C. Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus RCKK: A potential fungus. Bioprocess Biosyst. Eng. 2015, 38, 787–796. [Google Scholar] [CrossRef]
- Smith, D.C.; Bhat, K.M.; Wood, T.M. Xylan-hydrolysing enzymes from thermophilic and mesophilic fungi. World J. Microbiol. Biotechnol. 1991, 7, 475–484. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Z.Q.; Chi, Z.M. Production of phytase by a marine yeast Kodamaea ohmeri BG3 in an oats medium: Optimization by response surface methodology. Bioresour. Technol. 2008, 99, 6386–6390. [Google Scholar] [CrossRef]
- Tian, M.; Yuan, Q. Optimization of phytase production from potato waste using Aspergillus ficuum. 3 Biotech 2016, 6, 256. [Google Scholar] [CrossRef] [Green Version]
- Kornegay, E.T. Application of phytase for retention of nonphosphorus nutrients. Proc. MD Nutr. Conf. 1999, 46, 83–103. [Google Scholar]
- Simons, P.C.M.; Versteegh, H.A.J.; Jongbloed, A.W.; Kemme, P.A.; Slump, P.; Bos, K.D.; Wolters, M.G.E.; Beudeker, R.F.; Verschoor, G.J. Improvement of phosphorus availability by microbial phytase in broilers. Brit. J. Nutr. 1990, 64, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Kornegay, E.T.; Denbow, D.M.; Yi, Z.; Ravindran, V. Response of broilers to graded levels of microbial phytase added to maize-soybean-meal-based diets containing three levels of non-phytate phosphorus. Br. J. Nutr. 1996, 75, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Applegate, T.J.; Richert, B.; Angel, R. Phytase and Other Phosphorus Reducing Feed Ingredients; Fact Sheet AS-581-W; Purdue University Cooperative Extension Service: West Lafayette, IN, USA, 2008. [Google Scholar]
- Butler, T.A.; Sikora, L.J.; Steinhilber, P.M.; Douglass, L.W. Compost age and sample storage effects on maturity indicators of biosolids compost. J. Environ. Qual. 2001, 30, 2141–2148. [Google Scholar] [CrossRef]
- Chandra, R.; Yadav, S.; Kumar, V. Microbial degradation of lignocellulosic waste and its metabolic products. In Environment Waste Management; Chandra, R., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 250–293. [Google Scholar]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzyme: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Shahryari, Z.; Fazaelipoor, M.H.; Setoodeh, P.; Nair, R.B.; Taherzadeh, M.J.; Ghasemi, Y. Utilization of wheat straw for fungal phytase production. Int. J. Recycl. Org. Waste Agric. 2018, 7, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Choct, M. Enzymes for the feed industry: Past, present and future. Worlds Poult. Sci. J. 2006, 62, 5–15. [Google Scholar] [CrossRef]
- Sakpetch, P.; H-Kittikun, A.; Chandumpai, A. Isolation and screening of potential lignocellulolytic microorganisms from rubber bark and other agricultural residues. Walailak J. Sci. Tech. 2017, 14, 953–967. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanruean, K.; Penkhrue, W.; Kumla, J.; Suwannarach, N.; Lumyong, S. Valorization of Lignocellulosic Wastes to Produce Phytase and Cellulolytic Enzymes from a Thermophilic Fungus, Thermoascus aurantiacus SL16W, under Semi-Solid State Fermentation. J. Fungi 2021, 7, 286. https://doi.org/10.3390/jof7040286
Tanruean K, Penkhrue W, Kumla J, Suwannarach N, Lumyong S. Valorization of Lignocellulosic Wastes to Produce Phytase and Cellulolytic Enzymes from a Thermophilic Fungus, Thermoascus aurantiacus SL16W, under Semi-Solid State Fermentation. Journal of Fungi. 2021; 7(4):286. https://doi.org/10.3390/jof7040286
Chicago/Turabian StyleTanruean, Keerati, Watsana Penkhrue, Jaturong Kumla, Nakarin Suwannarach, and Saisamorn Lumyong. 2021. "Valorization of Lignocellulosic Wastes to Produce Phytase and Cellulolytic Enzymes from a Thermophilic Fungus, Thermoascus aurantiacus SL16W, under Semi-Solid State Fermentation" Journal of Fungi 7, no. 4: 286. https://doi.org/10.3390/jof7040286